4 THE NITROGEN CYCLE
advertisement

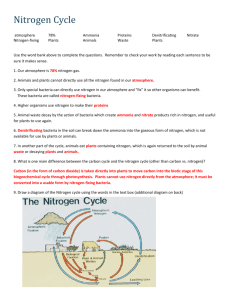
THE NITROGEN CYCLE I. Nitrogen pools and cycling times Cycling time refers to the average time an average nitrogen atom will remain unchanged in a given location Pool Cycling time Atmospheric Ammonia Days Oceans 7 weeks Forest Trees 16 years Atmospheric Nitrogen 44 Million years II. Biological uses of Nitrogen Nucleic Acids, which are made of nitrogen containing compound combined with a phosphate group and a sugar (N compound-CH8O2-PO4) Amino Acids are made a nitrogen containing amino group (NH2) combined with a carboxyl group (COOH) and one of 20 different side groups (R). The final product is: NH2-COOH-R. Amino acids combine to make proteins, enzymes, and hormones, which with carbohydrates are the major building blocks of all living organisms. The ratio of Nitrogen to Carbon demanded by organisms makes up part of a constant termed Redfield Ratios. These differ between aquatic and terrestrial systems. Habitat Optimum C:N ratio Land 106 C : 16 N Aquatic 106 C : 13 N Nitrogen requirements of aquatic species are less becasue they need less proteins to keep their body structure as they are partially held up by water. Land species require more proteins, as air support theur bodies much less than water. Because of the greater amount of proteins required by land species, Nitrogen is usually the element which will limit the vigour of their communities. III. The Nitrogen Cycle Step 1: Nitrogen-fixation Atmospheric: Happens when Nitrogen (N2) is oxidized at high temperatures (by lightning, in internal combustion engines) to make nitrite (NO2). This can combine with water to form nitric acid (H2NO3), which is deposited on earth through rainfall. Biological: Done by bacteria which can convert N2 into ammonia (NH3) if an energy source is present. Some get this energy by directly absorbing sunlight (blue-green algae) or by living in the roots of plants (legumes, alder trees), who provide them with food (Rhizobium, Azospirillium). Step 2: Conversion to Ammonia. As amino acids and nucleic acids require N in the form of Ammonia, if nitrate (NO3) present, it must be converted to NH3. This is done through Nitrate reductase enzymes. Step 3: Biological Use. Ammonia is incorporated into proteins, nucleic acids Step 4: When organism dies, ammonia is released back into the biosphere through the process of Ammonification, in which water is added to proteins to make carbon dioxide and ammonia. This process happens during digestion, and is also done by bacterial and fungal decomposers. Step 5: If ammonia released into oxygen rich (anaerobic) soil, other bacteria can convert it into nitrite or nitrate through the process of Nitrification: NH4+ + 2O2 = NO3- + H2O + 2H+. This is a problem, as it gives the molecule which contains Nitrogen a negative charge, which repels it from soil particles, causing it to be easily leached into streams and groundwater. Step 6: If soils remain anaerobic, another group of bacteria will convert it back into inert, atmospheric N2 through the process of Denitrification. In this process, bacteria use nitrate as an Oxygen source for respiration: C6H12O6 + 4NO3- = 6CO2 + 6H2O + 2N2 This is a problem, as any nitrogen returned to atmospheric will be stuck there for 44 million years until it is fixed again into a biologically useful form.