Pharmacy Medication Disposal SOP
advertisement

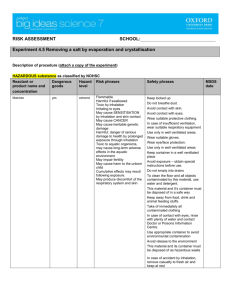
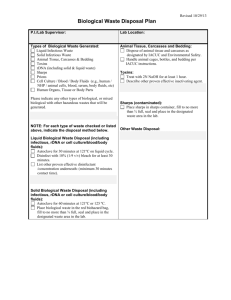
PHARMACY Standard Operating Procedure DISPOSAL OF MEDICATION Scope This procedure deals with the general medicine disposal. This SOP does not cover the disposal of Controlled Drugs. Disposal of Controlled Drugs is covered in the Pharmacy SOP: Controlled Drugs: Destruction Procedure P1 All staff are authorised to dispose of medicines All staff The following dispensary staff roles are authorised to deal with the disposal of medicines: Pharmacists Technicians Dispensers Healthcare Assistants P2 Quarantine medication Authorised staff Stock held under quarantine: P3 Securely seal stock in a bag and place in [insert details of designated] area clearly labelled with QUARANTINE STOCK. Add the date and reason why the stock has been segregated. Store quarantined stock in [insert details of designated area], away from all other stock, until further instruction is received. Fridge lines should be stored in [insert details of designated fridge] quarantine area. Expired or damaged stock – pharmaceutical waste Authorised staff P4 Log expired or damaged stock to be destroyed in [insert recording medium and location] Ensure date expired stock is clearly marked and stored separately from usable stock Sort waste according to the process outlined in P4 below Dispose of waste according to the process outlined in P5 below Sorting Authorised staff Wear appropriate protective clothing – gloves, apron and mask PHARMACY Standard Operating Procedure DISPOSAL OF MEDICATION P5 Identify any cytotoxic or other hazardous waste checking with the pharmacist where necessary. Identify any flammable waste and store separately until this can be removed by the waste contractor. Disposal Authorised staff If you have not already done so, wear appropriate protective clothing – gloves, apron and masks. For sharps: Do NOT remove needles from syringes – place the whole syringe into the sharps container Dispose of sharps in an appropriate sharps container – where possible it is good practice to use: [insert details of designated] sharps container for sharps contaminated with cytotoxic or cytostatic hazardous medicines [insert details of designated] sharps container for sharps contaminated with non-hazardous medicines [insert details of designated] sharps container for sharps which are not contaminated with medicines. For liquid medicines: Do not decant liquids from bottles into the waste container as the mixing of incompatible liquids into a single container could result in fire, release of fumes or explosion and harm to your staff or even prosecution could result. Empty bottles that have contained liquids should also be placed into the waste medicine container as they will contain a residue of medicine. For solid dosage forms: Do not ‘deblister’ i.e. remove individual tablets or capsules from blister packaging before placing the waste medicines into the waste disposal container. (This could be regarded as ‘waste treatment’ which could require a licence) For tablets or capsules contained within bottles – place the whole bottle into the waste container Where applicable remove the blister strips from the outer cardboard carton and place the intact blister strips into the waste container Cytotoxic and hazardous medicines or chemicals are disposed of in allocated bins as per guidance from waste contractor Dispose of gloves and apron if applicable Thoroughly wash hands. P6 Dealing with full containers PHARMACY Standard Operating Procedure DISPOSAL OF MEDICATION Authorised staff When full, seal the disposal container and store in a safe place, away from empty containers, ready for collection. Full disposal containers awaiting collection are located in the staff toilet. If the waste containers are full and the collection date is not in the near future, contact the scheme co-ordinator to arrange a collection. Make a record of the waste consignment in [insert name and location of recording medium] Complete the required information on the consignment note for hazardous waste. You can either list each item of hazardous waste individually or use a standard continuation sheet. Complete the waste transfer sheet (for non hazardous waste) Check that the number of containers has been correctly completed It is recommended that copies of all documentation supplied or completed by the approved waste collector, including the consignment note (for hazardous waste) and the waste transfer note (for non-hazardous waste) should be kept for a minimum of two years. I have read and understood this standard operating procedure. Date Date of Preparation: Name Date of next review: Prepared by: Signature: Date review takes Version: place and initials Signature DUE: