Study Guide answers for Test on Nov. 19
advertisement

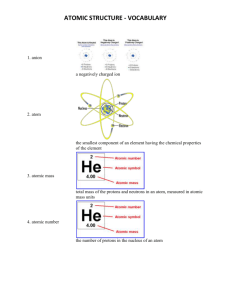
Chemistry Learning Targets 4 -7 Mastery Test Study Guide – Answer Key Target 4: Solutions and Mixtures 1. Define: solute The solute is part of the solution present in the lesser amount and dissolved by the solvent. 2. Give an example of a solute that can be mixed with water. An example of solutes would be kool-aid, salt, sugar, or oxygen. 3. Define: solvent The solvent would be the part of the solution present in the larger amount and dissolves the solute. 4. Give an example of a solvent. An example of a solvent would be water, or nitrogen 5. How do you determine if a mixture is homogenous? A mixture is homogeneous if the particles are so evenly distributed that you can’t see the different parts and it is not easily separated. 6. How do you determine if a mixture is heterogeneous? A mixture is heterogeneous if you can see the different parts of the mixture and it is easily separated out. Target 5: Elements and Compounds 7. Tell the difference between an element and a compound. An element is a pure substance made of only one kind of atom and it cannot be broken down into any other substance by physical or chemical means. A compound is a pure substance made of two or more elements chemically combined in a set ratio. 8. What is a symbol? Give an Example. A chemical symbol is used to represent an element. The symbol is single capital letter or a combination of a capital letter with one lower case letter. Examples of chemical symbols are: H – Hydrogen, He – Helium 9. What is a formula? Give an Example. A formula represents a compound. The formula is made up of at least two or more chemical symbols in a set ratio. Examples of a chemical formula would be: H2O, CO2 and NH4 10. Calculate the number of atoms of each element in a molecule/compound. H2O - 3 Atoms CO2 - 3 atoms C6H12O6 - 24 atoms Target 6: Atomic Theory 11. List the subatomic particles of an atom. – Protons, Electrons and Neutrons 12. Explain the charges associated with each subatomic particle. Protons have a positive charge and are located in the nucleus of the atom, Neutrons have no charge and are located in the nucleus of the atom, and electrons have a negative and are located orbiting the nucleus. 13. Draw and label a Carbon atom. (Label the subatomic particles) 14. How do you determine the atomic mass? The atomic mass is determined by adding the number of protons and neutrons together. 15. What subatomic particle makes up the atomic number? The subatomic particle that makes up the atomic number is the proton. Target 7: Radioactive Decay 16. Calculate how fast a radioactive isotope decays. The imaginary radioactive isotope Y has a halflife of 24 hours. What would the fraction of the original amount remaining be after 2 days? Explain your answer. (Show your reasoning.) 0 hours = 1/1 or 100% of the original element 24 hours = ½ or 50% of the original element 48 hours = ¼ or 25% of the original element Every 24 hours the radioactive isotope decays by half of the amount of the original element. After 48 hours ¼ of the original element is left because after 24 hours it decayed to ½ and then after another 24 hours, half of ½ is ¼. 17. Explain alpha decay. (what happens to atomic mass & atomic number?) The release of an alpha particle by an atom decreases the atomic number by 2 and the mass number by 4. For example: Thorium – 232 nucleus decays to produce an alpha particle and a radium -228 nucleus. 18. Explain beta decay. (what happens to atomic mass & atomic number?) During Beta decay, the neutron inside the nucleus of an unstable atom changes into a negatively charged beta particle and a proton. So the nucleus has one less neutron and one more proton. The mass number remains the same, but the atomic number increases by 1 19. Explain gamma decay. (what happens to atomic mass & atomic number?) During gamma radiation, high energy waves are emitted, similar to X-Rays. Gamma radiation has no charge and does not cause a change in either the subatomic mass or the atomic number.