SUPPLEMENTARY MATERIAL Study of the Effect of Nickel Heavy
advertisement

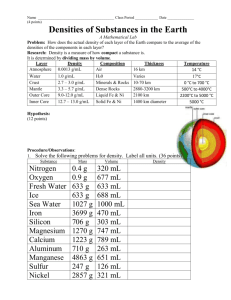
SUPPLEMENTARY MATERIAL Study of the Effect of Nickel Heavy Metals on Some Physiological Parameters of Catharantus roseus Matin Arefifard, Majid Mahdieh Mohammadreza Amirjani Biological Department, Arak University, Iran Plants, in their life cycle, are usually exposed to various kinds of non-biological stresses including heavy metals. One of these heavy metals is nickel which affects many physiological processes of plants. Studies have shown that change in the planting conditions can affect the qualitative and quantitative features of Catharantus roseus; therefore, creating stressful conditions (e.g., NiCl2) can be an effective way to investigate the changes. In this research, we investigated effect of 0, 2.5, 5, 10, 25, 50 mM density of NiCl2 on degree of catalase enzyme activity, amount of Prolin aggregation, and photosynthetic parameters on seeds of pink variety of Catharantus roseus. The results showed that the degree of catalase enzyme activity and the amount of prolin aggregation increased in plants which were under NiCl2 treatments, especially in high densities, while the total protein decreased. The stress of Ni also affected photosynthetic parameters, and decreased the amount of pigments, as well as the photosystem II efficiency. Keywords: Catharantus roseus, Nickel heavy metal, Catalase, Photosynthetic parameters Experimental Cultivation The C. roseus healthy seeds of pink variety were sterilized using Sodium Hypochlorite of 1%, for 10 min, Ethanol 70%, for 5 min, Mercury Chloride 0.2% for 10 min. Then the seeds were washed three times using triple distillation water (TDW). Ten seeds were planted, in vases and irrigated with Hoagland solution of 50%, and pH=5.8, and kept in a planting room with photo periods of 16:8 L:D, with relative humidity 30 to 40% and temperature was 28+2 ˚C and 25+2 ˚C during light and dark period, respectively. The surface of each vase was covered by nylon plastic till the time of germination. In week seven, the plants of the same size were . Corresponding Author. Email: matinarefifard@yahoo.com extracted unharmed and were put in vials while their ends were cut. Their roots were planted in 50% Hoagland solution. The change of planting environment was done every four days, and daily ventilation was done for four hours by an air pump. After a week, the 45-day-old plants were exposed to Nickel chloride stress with densities of 0 (control), 2.5, 5, 10, 25, 50 mM for 96 h (three replicate for each treatment). Measurement of Proline Amino Acid Fresh tissue (0.3 g) of the plant shoot was pulverized in 3% Sulfo-salicylic acid. The obtained solution was centrifuged, and 400 μl of the transparent solution was added to 2 ml of glacial acetic acid along with 2 ml of Ninhydrin (1.25 g of Ninhydrin plus 30 ml of glacial acetic acid and 20 ml of 6 molars phosphoric acid). The resulted mixture was put in the test tube in order to be heated for one hour in the hot bath (100 ˚C) until it became entire solution. The heat made amino acid and Ninhydrin react. The reaction ended in ice water bath. Later, 4 ml of Toloen was added to the solution, and the resulted liquid was strongly shaken for 20 seconds. The test tubes were kept consistent for one hour in order to separate the two phases. The organic pink phase stood at the top, and the colorless and transparent phase stood at the bottom. The organic phase was used to measure the colors. The spectrophotometer (Pharmacia LKB-Novaspec Model) was adjusted in wave length of 520 nm with toloen, and then the absorption intensity of each sample was read. The amount of proline in the given tissue was calculated based on the proline standard curve in the densities of 0, 20, 40, 60, 80, and 100 ppm in μg/g (Baste et al., 1973). Measurement of Catalase Enzyme Activity Preparation of Enzyme Essence Frozen leaf (0.1 g) in -80 ˚C was ground in 1 ml of sodium phosphate buffer 0.05 M (PH=7.8) containing one mM of EDTA in the frig. The obtained solution was centrifuged for 20 min in the temperature of 4 ˚C with the speed of 13000 rpm, and then the solution which remained at the top was transferred to new vials where it was used for determining the total amount of protein and enzyme activity. Determining the Amount of Total Protein The necessary solutions for this method are the Loari work solution, and the standard protein solution given below: Solution A: Sodium carbonates 2% NaOH 0.1 M Solution B: Tartrate sodium potassium 2% Solution C: Zinc sulfate 1% Solution D: Foline indicator which is diluted by distilled water with the ratio of 1 to 1 The Loari work solution: 98 ml of Solution A+1 ml Solution B+1 ml of Solution C For standard protein solution, one mg of bovine serum albumin powder (BSA with molecule weight of 66.2 kD) is solved in 5 ml of distilled water densities of 20, 40, 60, 0, 100 μg protein are used for drawing the curve. In this method, the Peptidic link of protein with zinc Sulfate produces a blue color. Aromatic amino acids of tyrosine and tryptophan in protein with Phospolipidic acid and tungsten in Loari indicator produces blue color complex. The amount of color (appropriate for the protein density), was added to clean test tubes containing unknown samples and standard solutions, as well as, 5 ml of Loari work solution, and 20 μl of extracted essence from the plant leaves. After 5 min, all test tubes including unknown and standard solutions were added by distilled water to reach the same volume, and then 0.5 ml of solution D was added to all of them. After 15 min, the amount of absorption of samples was recorded in the wavelength of 750 nm. Using the standard curve of protein, the protein density of extracted essences was calculated. Catalase Enzyme activity The rate of use of H2O2 in 100 μl of enzyme essence along with 2 ml of buffer solution of phosphate sodium 25 mM (pH=7) containing H2O2 10 mM was calculated using photometry method in wavelength of 240 nm for 30 seconds. The spectrophotometer changed to zero with the buffer of phosphate sodium of 25 mM free of H2O2. Then, 2 ml of buffer containing H2O2 was poured and 100 μl of enzyme essence kept in the ice was mixed with buffer and the absorption was recorded immediately. Without taking the sample out of the machine, after 30 s, the secondary absorption of the sample was recorded. The off coefficient of H2O2 is 0.039. Regarding this coefficient, the coefficient of the enzyme activity was calculated based on H2O2 μm per min in total mg protein. Leaf Chlorophyll Frozen leaf (0.1 g) in -80 ˚C was ground along with 2 to 3 ml of acetone 80% in darkness in a clean in a mortar. After filtering the solution, the volume increased to 10 ml by adding acetone. Then, the absorption of the solution was recorded at wavelength of 645, 652, and 663 nm using spectrophotometer. The amount of chlorophyll a and b and the total chlorophyll was estimated based on milligram chlorophyll per each gram of the leaf tissue using the following formulas: Formula 1 Chlorophyl a= [12.7A663 -2.69A645 ]×(V/1000)×W Formula 2 Chlorophyl b = [22.9A645 -4.68A663 ]×(V/1000)×W Formula 3 Total Chlorophyl = [20.2A645 -8.02A663 ]×(V/1000)×W Maximum Efficiency of Photo System II (Fv/Fm) The 45-day-old plants affected by the florescence ratio of variable (Fv) to maximum florescence (Fm), were calculated. The plant was kept in darkness for 30 m in order to adapt it. Then, in the same darkness condition and using mini pam machine, WALZ model, the maximum and basic florescence was recorded. The variable florescence equals maximum florescence minus basic florescence. YIELD=Fm-m/Fm=Fv/Fm Statistical analysis The statistical design was completely random. The drawing of graphs was done by EXCEL and the analysis of factors by SPSS software, V11. The Duncan method was applied in order to compare the means of the obtained data and one way ANOVA. References: Abdul Jaleel, C., Gopi, R., Manivannan, P. and Panneerselvam, R. (2008) Soil salinity alters the morphology in Catharanthus roseus and its effects on endogenousmineral constituents. EurAsian Journal of BioSciences 2: 18-25. Abdul Jaleel, C., Manivannan, P., Lakshmanan, G. M. A., Gomathinayagam, M. and Panneerselvam, R. (2008) Alterations in morphological parameters and photosynthetic pigment responses of Catharanthus roseusunder soil water deficits. Colloids and Surfaces B: Biointerfaces. 61: 289-303. Ataei-Azimi, A., Delnavaz Hashemloian, B., Ebrahimzadeh, H. and Majd, A. (2008) High in vitro production of antcanceric indole alkaloids from periwinkle (Catharanthus roseus) Tissue culture. African Journal of Biotechnology 7(16): 2834-2839. Babaglui. S., Kusvuran, S.,Ellialtioglu, S.(2008) Antioxidative Enzyme response of heavy metal hyperaccumulator alyssum murale to Ni+ stress. Physiol Plant 15:89-94. Chen, C., Huang D., Liu, J. (2009) Functions and Toxicity of Nickel in Plants. Recent Advances and Future Prospects 37: 304-313. Croteau, R., Kutchan, T. M. and Lewis, N. G. (2000) Natural products (secondary metabolites). In Biochemistry and molecular biology of plants: 1250-1318. Rockville, M. D. American Society of Plant Physiologists. Felipe Vázquez-Flota, M. C. P. and M. d. L. M. H. Yereni Minero-García (2004) Alkaloid metabolism in wounded Catharanthus roseus seedlings. Plant Physiology and Biochemistry 42: 623-628. Filippini, R., Caniato, R., Piovan, A. and Cappelletti, E. M. (2003) Production of anthocyanins by Catharanthus roseus. Fitoterapia 74: 62–67. Kaminaga, Y., Nagatsu, A., Akiyama,T., Sugimoto,N., Yamazaki,T., Maitani,T., Mizukami,T. (2003) Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. Phytochemistry 12: 64-70. Paulo, R., Moreno, H.(1995) Effects of elicitation on different metabolic pathways in Catharanthus roseus (L.)G.Don cell suspension cultures. Plant Physiology 112:10–17 Shanks, J. V., Bhadra, R. and Morgan, J. (1998) Effect of salinity and different nitrogen sources on the activity of antioxidant enzymes and indole alkaloid content in Catharanthus roseus seedlings. Journal of Plant Physiology163:11-18 Smirnoff, N. (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27-58. van Bergen, M., Snoeijer, W. (1996) Catharanthus G.Don. The Madagascar Periwinkle and Related Species. Wageningen Agricultural University 96: 1-120. Van Breusegem, F., Vranova, E., Dat, J. F. and Inze, D. (2001) The role of active oxygen species in plant signal transduction. Plant Sci 161: 405– 414. 120 a 100 b Proline (µg/g FW( 80 c 60 40 d d 20 e 0 0mM 2.5mM 5mM 10mM 25mM 50mM Densities of Nickel Chloride Figure S1. The effect of different densities of NiCl2 on the amount of proline accumulation the plant shoot µmol/min.mg protein) use of H2O2 400 350 300 250 200 150 100 50 0 b ab b b mM0 mM2.5 mM5 a b mM10 mM25 mM50 Densities of Nickel Chloride Figure S2. The effect of different densities of NiCl2 on the catalase enzyme activity 3 e e 2.5 mg/gFW 2 d e d Chla 1.5 1 c c d d b Chlb a a c b ab 0.5 b a ChlT a 0 0mM 2.5mM 5mM 10mM 25mM 50mM Densities of Nickel Chloride Figure S3. The effect of different densities of NiCl2 on chl"a". "b"and total chlorophyll 3 a b 2.5 bc chla/chlb 2 bc c c 1.5 1 Chla 0.5 0 0mM 2.5mM 5mM 10mM 25mM 50mM Densities of Nickel Chloride Figure S4. The effect of different densities of NiCl2 on chl "a" / "b" Fv/Fm 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 a ab b c mM0 mM5 mM25 mM50 Densities of Nickel Chloride Figure S5. The effect of different densities of NiCl2 on the efficiency of PSII 6 a 5 total protein 4 b 3 c d 2 d e 1 0 0mM 2.5mM 5mM 10mM 25mM 50mM Densities of Nickel Chloride Figure S6. The effect of different densities of NiCl2 on the total amount pf protein