Heat energy - review questions
advertisement

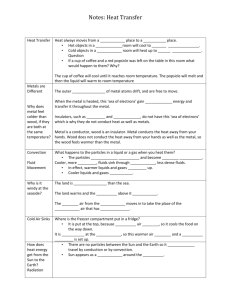
Heat Energy - Review questions - 1. Arrange the following temperatures descending order: 75°C, 100°C, -25°C and 0°C 100°C, 75°C, 0°C, -25°C 2. Copy and complete these sentences a. Temperature is a measure of how hot something is. b. Temperature is measured with a thermometer. c. On the Celsius scale, water boils at 100°C and freezes at 0°C. d. Metals are good conductors of heat. e. Plastics are good insulators because they prevent heat from passing through them. f. Heat can travel through solids because the particles in them bump into each other. g. As an object gets hotter, its particles move faster. 3. Why are you more likely to burn your lips drinking from a metal cup than you are drinking from a plastic cup? You are more likely to burn your lips drinking from an iron cup because unlike the plastic cup that is a good insulator, the metal cup conducts heat. The heat can travel easily from the liquid through the cup and burn your lips. 4. You get into a bath containing water at 45°C. a. What will happen to your body temperature? Your body temperature would increase, trying to match the temperature of the water. b. What will happen to the temperature of the water? The temperature of the water would decrease, trying to match the temperature of your body. c. Does heat flow from the water into your body or from your body into the water? Explain. As the water has more heat energy than your bogy holds, the heat from the water would transfer to your body, so that your body and the water are at equilibrium. 5. You leave the door of your heated house open on a cold night. Does the cold come in or does the heat go out? Explain. Hot air will leave, and enter into the cooler area through conduction; the temperature wants to reach symmetry with the surrounding air. 6. a. Which is hotter – a cup of water at 50°C or a bath full of water at 50°C? Temperature is a measure of how fast the atoms move, and as the atoms are moving at exactly the same speed, they are both the same temperature. However, since there are more molecules in the bathtub that contain heat energy, the water in the bathtub holds more heat energy then that of the cup. b. Which contains more heat energy – the cup of water or the bath full of water? It depends on the amount of kinetic energy that the molecules hold. Technically speaking, the bathtub is more likely to hold more heat energy, as there are more molecules. 7. Your friend Kate says that the iron in fence feels hotter than the wooden posts. How could you convince her that the iron and the wood are really at the same temperature, and explain to her why the iron feels hotter? Your sense of an object’s temperature has to do with the ability of that object to conduct its heat from itself to your skin. The iron is a really good conductor, and so you can feel the heat to touch really easily. As your hand is cooler than the iron, heat naturally flows from the iron to your hand as to reach a point of equilibrium. The wood on the other hand, is a poor conductor, and so the heat does not transfer as easily, and it feels cooler to touch. The wood would not be able to quickly transfer its heat energy to your hand like the iron did, and so it feels cooler to touch. 8. Use what you have learnt to explain each of the following: a. Containers (e.g. a saucepan) generally have plastic handles. Saucepans would more often than not have a plastic handle. This is because plastic is a good insulator, and the metal is a good conductor. To stop your hand fro getting burnt from direct contact with the metal, you cover the metal with something that will stop or slow down heat transfer between the handle and your hand. So you cover the metal with plastic and you are less likely to be burnt. b. Putting a metal skewer into a potato will decrease the time needed to bake it. By placing the metal skewer in the potato when you bake it allows the heat to travel through the conductor easily, the conductor being the skewer, and directly to the middle of the potato, allowing heat to penetrate the potato, and cook it on the outside and the inside at the same time. c. It is warmer to wear to thin jumpers than one thick one. It is warmer to wear thin jumpers because layering your insulators traps stagnant air and heat energy better, keeping it close to your skin and not allowing to escape. d. Drink coolers are made from foam plastic rather than solid plastic. Styrofoam is a better insulator than polystyrene Styrofoam is expanded polystyrene, it has pockets of stagnant air trapped within it. Air, and gases in general, are very poor conductors as the transfer of heat energy relies on friction and the particles in gases are further apart then that of solids and liquids, making it harder for heat energy to bump into and transfer. The Styrofoam cup is very resistant to heat transfer. The winding chain of molecules on the inside of the cup, as a result from blowing the polystyrene up into a foam, makes it harder for heat to just go through smoothly, it has to follow a winding set of molecular structures. 9. What is convection? Convection is the travel of heat energy as it goes with the flow of another substance ie. Liquid or gas. 10. Suggest why the air in a heated room is usually cooler near the floor than near the ceiling. Hot air rises, because the molecules are further apart and vibrate more rapidly than that of cool air, allowing the hot air to work its way to the top of the room. In a heated room, the floor will be cooler and the roof warmer because of this principle. 11. a. Where is the cooling element in a refrigerator? Why? The cooling system in a refrigerator is at the top. It is always placed at the top, because hot air naturally rises and the cool air that it is pumping out will naturally fall to the bottom of the fridge. The hot warmer air will rise, but because the cooling system is at the top of the fridge, it pumps out more cool air, making the top the most effective place to put the cooling system. b. What happens to the air inside a fridge when you open the door? Why? Heat energy travels via convection and makes the cool air in the fridge warmer. This is because heat energy naturally travels to cooler places, not warmer. 12. Draw a diagram showing how a hot cup of coffee cools by convection. 13. If you are cooking a dish that needs a very high temperature, the recipe may tell you to put it on the top shelf of the oven. Explain. The recipe may tell you to put it on the top shelf of the oven because hot air rises, and it will be exposed to more heat on the top shelf then on other shelves. 14. Glider pilots look for thermals. What are they and why do gliders need them? Thermals are convection currents, where heat travels along. Gliders use them because they can catch them, and it’s hot air rising, and it’ll allow them to stay suspended in the air for longer with minimal effort. 15. Suppose you are camping and you want to boil a billy on a fire. Would you place the billy on the ground and build the fire around it, or would you hang the billy from above the fire? Explain your answer. I would put the billy on top of the fire, because hot air rises and the kettle would be hit with the full heat of the fire. 16. Suppose you have two surfaces, one white and one black. a. Which is the better radiator of heat? Black b. Which is the better reflector? White c. Which is the better absorber? Black 17. The diagram below shows an experiment to measure how well six different materials conduct heat. Use the results to help you answer the following questions: a. Which material is the best conductor? How do you know this? The copper is the best conductor, and we can tell because it is the one with the least wax. b. Which material is the best insulator? The wax is the best insulator. c. Would food cook more quickly in a glass or an iron casserole dish? Food would cook faster in an iron casserole dish. d. Why do some saucepans have copper bases? Because copper conducts heat really well, and it would allow heat energy to be transferred from the heat source to the food faster. e. Is water more likely to freeze in a plastic or a copper pipe? Explain. It is more likely to freeze in a plastic pipe, because the iron would conduct heat to the water, and plastic would stop it from getting through as easily.