J Topic 9 notes - The University of West Georgia
advertisement

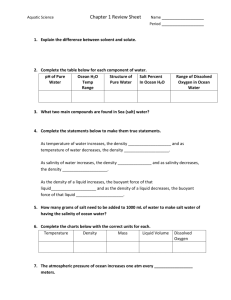
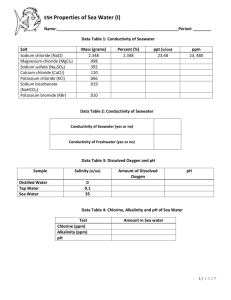
GEOL 2503 Introduction to Oceanography Dr. David M. Bush Department of Geosciences University of West Georgia Topic 9: Salt Water POWERPOINT SLIDE SHOW NOTES 1 2 Topic 9: Salt Water What is in Seawater? Salinity. Salinity is a measure of the amount of dissolved solids in seawater. We often refer to the dissolved solids simply as “salt” because the ocean is salty. But there is more than just true salt (table salt) dissolved in sea water. Also, here we are talking about dissolved solids. We’ll talk about gases (such as oxygen) a little later. 3 4 5 6 7 8 9 10 11 12 13 14 Note the fancy “‰” symbol. Like a percent sign with an extra 0 on the bottom. This is the symbol for “per mille” meaning per thousand. Average seawater salinity is 35 parts per thousand, or 35‰. Thus, a kilogram of seawater (one thousand of the grams, or cubic centimeters of water), contains 35 grams of dissolved solids. All naturally occurring solids can be found dissolved in seawater, but only six elements are found in abundance everywhere. These are called the major constituents. Dominating the major constituents are sodium and chlorine which together make up table salt. The six major constituents make up 99.36% of everything dissolved in seawater. The rest are called trace elements. The constituents of seawater. Note that the major constituents are shown in some different forms here than in the previous slide, but the basic elements are the same. The actual amounts of the major constituents that are found in one kilogram of seawater. The major constituents add up to 34.91 grams out of the 35 grams of solids dissolved in seawater. Note that chlorine and sodium (NaCl—table salt) add up to almost 86% of the major constituents. Another way to look at the major constituents. Note that again, the forms of the elements are a little different than the way they were introduced. For example, chloride is just chlorine with an extra electron; sulfate is just sulfur with oxygen bonded to it. Table salt Just like solid water had a definite crystal structure, so does table salt when it is in crystal form. Table salt has the chemical name of sodium chloride. The negative chlorine atoms bond to the positive sodium atoms. Halite dissolves easily in water. The molecules break apart, or dissociate. The sodium and chlorine break apart and are surrounded by water molecules. Because of its polar nature, water dissolves everything given enough time. That is why water is called the universal solvent. Also, the dissolving ability of water means that minerals dissolved by rivers and ground water on land eventually make their way to the ocean. These dissolved solids accumulate in the ocean to become the salt in seawater. Remember, we use the term “salt” in the ocean to mean all dissolved solids, not just the mineral halite. The oceans haven’t gotten saltier for billions of years because processes that add salt to the ocean (inputs) are balanced by processes that remove salt (outputs). Salt inputs and outputs. 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 Principle of Constant Proportions. Applies only to major constituents. Also known as Forchhammer’s principle. Salinity varies, meaning total amount of salt varies, 34‰, 35‰, 36‰, etc. But the ratios stay the same. There is always 55.07% chlorine, 30.62% sodium, and so forth. Constant proportions makes it much easier to measure salinity. Instead of having to obtain a measured sample of seawater (1 kg, for example), letting the water evaporate, and weighing the solids left behind, we only need to measure the amount of one of the major constituents and then we can calculate the total amount of dissolved solids. Most salinometers simply measure the amount of dissolved chlorine. Gases are also dissolved in seawater. They do not contribute to salinity, which is only dissolved solids. We’ll look at only three common gases. Open a can of soda and you’ll release pressure and carbon dioxide (the carbonation) will fizz out of the soda. Releasing pressure means the soda can’t keep as much carbon dioxide dissolved. Also, try it with a warm soda versus a cold one. The cold one fizzes a lot less than the warm one. Warm water can’t hold as much carbon dioxide dissolved in it. Oxygen in the ocean Carbon dioxide in the ocean Carbon dioxide and oxygen graphed versus depth in the ocean. Note how different the two curves are. Oxygen concentration in the ocean varies with depth. Why does oxygen concentration increase below 800 meters? It is only formed at the surface, so how can it increase? Carbon dioxide concentration in the ocean varies with depth. Salt as a resource Desalinization is also known as desalination. Why desalinate? Solar still A solar still example Osmosis Reverse osmosis can be used to desalinate water. Reverse osmosis desalinization. We supply pressure and push salt water through a semipermeable membrane which filters out the salt and provides fresh water. These canisters hold the filters in a reverse osmosis operation. The canisters must be cleaned occasionally to remove material from the filters.