Minerals and Their Properties
advertisement
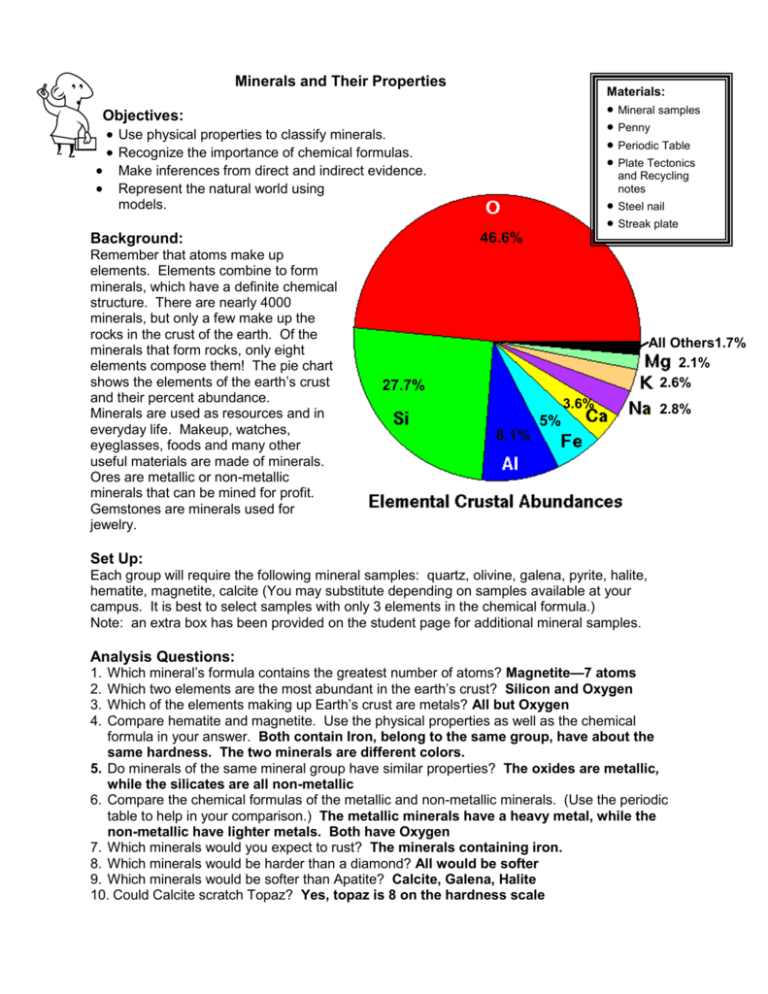
Minerals and Their Properties Materials: Mineral samples Penny Periodic Table Plate Tectonics Objectives: Use physical properties to classify minerals. Recognize the importance of chemical formulas. Make inferences from direct and indirect evidence. Represent the natural world using and Recycling notes Steel nail Streak plate models. 46.6% Background: Remember that atoms make up elements. Elements combine to form minerals, which have a definite chemical structure. There are nearly 4000 minerals, but only a few make up the rocks in the crust of the earth. Of the minerals that form rocks, only eight elements compose them! The pie chart shows the elements of the earth’s crust and their percent abundance. Minerals are used as resources and in everyday life. Makeup, watches, eyeglasses, foods and many other useful materials are made of minerals. Ores are metallic or non-metallic minerals that can be mined for profit. Gemstones are minerals used for jewelry. All Others1.7% 2.1% 2.6% 27.7% 3.6% 5% 2.8% 8.1% Set Up: Each group will require the following mineral samples: quartz, olivine, galena, pyrite, halite, hematite, magnetite, calcite (You may substitute depending on samples available at your campus. It is best to select samples with only 3 elements in the chemical formula.) Note: an extra box has been provided on the student page for additional mineral samples. Analysis Questions: Which mineral’s formula contains the greatest number of atoms? Magnetite—7 atoms Which two elements are the most abundant in the earth’s crust? Silicon and Oxygen Which of the elements making up Earth’s crust are metals? All but Oxygen Compare hematite and magnetite. Use the physical properties as well as the chemical formula in your answer. Both contain Iron, belong to the same group, have about the same hardness. The two minerals are different colors. 5. Do minerals of the same mineral group have similar properties? The oxides are metallic, while the silicates are all non-metallic 6. Compare the chemical formulas of the metallic and non-metallic minerals. (Use the periodic table to help in your comparison.) The metallic minerals have a heavy metal, while the non-metallic have lighter metals. Both have Oxygen 7. Which minerals would you expect to rust? The minerals containing iron. 8. Which minerals would be harder than a diamond? All would be softer 9. Which minerals would be softer than Apatite? Calcite, Galena, Halite 10. Could Calcite scratch Topaz? Yes, topaz is 8 on the hardness scale 1. 2. 3. 4. __Calcite__________ Formula: CaCO3 Hardness: 3.0 Cleavage: yes Luster: non-metallic Streak: white Mineral Group: carbonates ____Galena__________ Formula: PbS Hardness: 2.5 Cleavage: yes Luster: metallic Streak: gray/black Mineral Group: Sulfites ____Pyrite__________ Formula: FeO2 Hardness: 6.5 Cleavage: no Luster: metallic Streak: green/black Mineral Group: oxides ____Olivine__________ Formula: Mg2SiO2 Hardness: 6.5-7.0 Cleavage: yes Luster: non-metallic Streak: white Mineral Group: silicates ____Halite__________ Formula: NaCl Hardness: 2.5 Cleavage: yes Luster: non-metallic Streak: white Mineral Group: salts ____Magnetite__________ Formula: Fe3O4 Hardness: 5.5-6.0 Cleavage: no Luster: metallic Streak: black Mineral Group: oxides ____Hematite__________ Formula: Fe2O4 Hardness: 6.5 Cleavage: no Luster: metallic Streak: red/brown Mineral Group: oxides ____Quartz__________ Formula: SiO2 Hardness: 7.0 Cleavage: no Luster: non-metallic Streak: white Mineral Group: silicates ______________ Formula: Hardness: Cleavage: Luster: Streak: Mineral Group: Minerals and Their Properties Objectives: Use physical properties to classify minerals. Recognize the importance of chemical formulas. Make inferences from direct and indirect evidence. Represent the natural world using models. Background: Remember that atoms make up elements. Elements combine to form minerals, which have a definite chemical structure. There are nearly 4000 minerals, but only a few make up the rocks in the crust of the earth. Of the minerals that form rocks, only eight elements compose them! The pie chart shows the elements of the earth’s crust and their percent abundance. Minerals are used as resources and in everyday life. Makeup, watches, eyeglasses, foods and many other useful materials are made of minerals. Ores are metallic or non-metallic minerals that can be mined for profit. Gemstones are minerals used for jewelry. 46.6% All Others1.7% 2.1% 2.6% 27.7% 3.6% 2.8% 5% 8.1% Procedure: 1. Construct a model to represent a molecule of each mineral sample. Do this by using the chemical formulas for each mineral in Table A. Use a penny as a template for each atom. Color each element a different color. 2. Write the name of each mineral and its chemical formula in the spaces provided in each box. 3. Use your notes, a steak plate and the field hardness test kit to determine the physical properties of each mineral sample. Table A Mineral Name Calcite Corundum Galena Halite Hematite Magnetite Olivine Pyrite Quartz Chemical Formula CaCO3 AlO3 PbS NaCl Fe2O3 Fe3O4 Mg2SiO2 FeS2 SiO2 Corundum Formula: Al2O3 Hardness: >7 Cleavage: no cleavage Luster: nonmetallic Streak: no streak Al O Analysis Questions: ______________ Formula: Hardness: Cleavage: Luster: Streak: ______________ Formula: Hardness: Cleavage: Luster: Streak: ______________ Formula: Hardness: Cleavage: Luster: Streak: ______________ Formula: Hardness: Cleavage: Luster: Streak: ______________ Formula: Hardness: Cleavage: Luster: Streak: ______________ Formula: Hardness: Cleavage: Luster: Streak: ______________ Formula: Hardness: Cleavage: Luster: Streak: ______________ Formula: Hardness: Cleavage: Luster: Streak: : ______________ Formula: Hardness: Cleavage: Luster: Streak: 1. Which mineral’s formula contains the greatest number of atoms? 2. Which two elements are the most abundant in the earth’s crust? 3. Which of the elements making up Earth’s crust are metals? 4. Compare hematite and magnetite. Use the physical properties as well as the chemical formula in your answer. 5. Do minerals of the same mineral group have similar properties? 6. Compare the chemical formulas of the metallic and non-metallic minerals. (Use the periodic table to help in your comparison.) 7. Which minerals would you expect to rust? 8. Which minerals would be harder than a diamond? 9. Which minerals would be softer than Apatite? 10. Could Calcite scratch Topaz?








