Review Filled In
advertisement

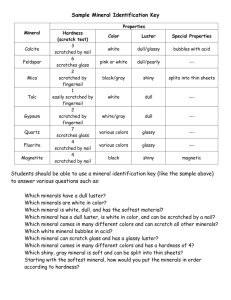
Unit 2 TEK 6.6 A, 6.6B, 6.6C Review TEK 6.6A- Metals, Nonmetals & Metalloids Properties of Metals: 1. 2. 3. 4. 5. Shiny (Luster) Good conductor of electricity and thermal energy Malleable (Hammered into sheets) Ductile ( pulled into wires) Mostly solid at room temp Uses of metals: 1. 2. 3. 4. 5. Jewelry Vehicles Thermometer Coins Technology Properties of Nonmetals: 1. 2. 3. 4. 5. No Luster Poor conductor of electricity and thermal energy Good Insulator Brittle (NOT Malleable or Ductile) Many are gases at room temp Uses of Nonmetals: 1. Insulator 2. Nose cones on space shuttles 3. Diamonds, graphite(pencils) Properties of Metalloids: 1. Has physical and chemical properties of both metals and nonmetals 2. Act as a semiconductor ( Good conductor at high temps) Uses of metalloids: 1. 2. 3. 4. Fireworks Water softeners Laundry products Computer chips TEK 6.6B- Density Define the following: Density- The mass per unit volume of a substance Mass- The amount of matter in an object Volume – The amount of space an object takes up What are the units for density? What is the formula for density? - mass ÷ volume Practice Problems: 1. An irregularly shaped stone was lowered into a graduated cylinder holding a volume of water equal to 2 ml. The height of the water rose to 7 ml. If the mass of the stone was 25 g, what was its density? D= D= D= D= 2. A piece of wood that measures 3.0 cm by 6.0 cm by 4.0 cm has a mass of 216 grams. What is the density of the wood? (volume = L x W x H) D= D= D= D= 3. What is the density of on object with a mass of 20g and a volume of 7mL? D= D= D= D= 4. An irregularly shaped stone was lowered into a graduated cylinder holding a volume of water equal to 20.0mL. The height of the water rose to 30.0 mL. If the mass of the stone was 60g, what was its density? D= D= D= D= 5. Calculate the density of a 600 g rectangular block with the following dimensions: length=8 cm, width=5 cm, height=5 cm. D= D= D= D= TEK 6.6C- Minerals Minerals are: 1. Naturally occurring a. Formed in processes on or in the earth with no human input 2. Inorganic a. Not made by living process b. *It has never been alive! 3. Definite chemical composition a. All minerals are elements or compounds with definite chemical composition 4. Crystal Structure a. The atoms of the mineral are arranged in patterns that repeat over and over again. b. Ex. Graphite versus Diamond The 5 physical properties we use to identify minerals are (be sure to explain each): 1. Color This is an easy clue, but it can be misleading. Many different minerals have the same color and appearance… ex) pyrite and gold 2. Hardness A measure of how easily a mineral can be scratched. Mohs Hardness Scale 3. Luster The way the mineral reflects light. Either metallic or non-metallic. Nonmetallic can be: Dull, Pearly, Silky, and Glassy 4. Streak The color of the mineral in the powdered form. 5. Cleavage and Fracture Cleavage – mineral that break along a smooth, flat surface (ex. Mica) Not all minerals have cleavage… Fracture – minerals that break with uneven, rough, or jagged surfaces (ex. Halite) Answer the following questions using Moh’s hardness scale: 1. Which mineral could be scratched by a copper penny but not by a fingernail? Calcite 2. Which mineral can be scratched by Quartz but no by Apatite? Feldspar 3. Which minerals can be scratched by Topaz but not by Flurite? Quartz, Feldspar, and Apatite Use the following chart to answer the questions below: Observation of Unknown Substance Unknown Conducts Electricity Luster Malleability 1 2 3 No Yes No Dull Shiny Shiny Snaps easily into small pieces Bends easily Bends and snaps back to original shape 4 Yes Dull Shatters easily 1. Which of the unknown substances is most likely a metal? __2___ 2. Which of the unknown substances is most likely a metalloid? ___3&4___ 3. Which of the unknown substances is most likely a nonmetal? ___1___