The Evolutionary Analysis of Cytochrome P450
advertisement

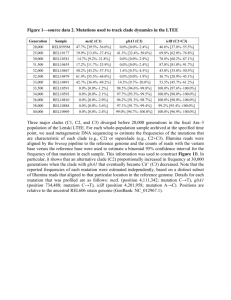
The Evolutionary Analysis of Cytochrome P450 in Aquatic Fish Species Using Phylogenetic Analysis Devin Radel Jett Peng This analysis was co-conducted throughout the entire process. Introduction: For our research we decided to examine the evolutionary relationships between different aquatic fish species from the analyzation of cytochrome p450. Cytochrome p450 is involved with the metabolism of foreign compounds like pollutants. We are examining different aquatic species from different habitats (lakes, freshwater streams, the ocean and its coastal environments) to infer phylogenetic relationships of cytochrome p450 among those species. This research can lead to further examination and monitoring of these waterways to examine possible mutations in the species present there. By doing this we may find areas of concern in aquatic environments to infer possible pollution events, since pollution itself cannot be directly inferred from the obtained data. Cytochrome P450 (CYP) is an enzyme that is responsible for the metabolism of foreign compounds or xenobiotics (environmental contaminants) (Harskamp). An environmental contaminant can be any foreign substance that is introduced into an environment by pollution, run-off, or any other anthropogenic means. Environmental pollutants can harm organisms in many different ways; the most common is through hormonal responses, which are more commonly referred to as Endocrine Disruptors (Kloas). These toxins (Endocrine Disruptors) are harmful to aquatic organisms due to their ability to inhibit cytochrome P450 causing the organism to be susceptible to the effects of the toxin (Kloas). Due to this great threat organisms in aquatic environments must adapt by means of mutation or selection to ensure their survival. In a previous study conducted by John Stegeman and Pamela Kloepper-Sams entitled “Cytochrome P-450 Isozymes and Monooxygenase Activity in Aquatic Animals” researchers examined the mechanisms and functionality of cytochrome P450 when introduced to different environmental toxins (Steggman). It was claimed that cytochrome P450 activity was most observable in fish species. In this study it was found that cytochrome P450 responds similarly to these toxins in all examined species (Rainbow Trout, Scup, and Cod) (Steggman). As suggested above, this research examines the biochemistry behind the mechanism of cytochrome P450 to determine its function in an organism with relation to multiple terrestrial and aquatic species (Steggman). Our research will be a supplement to this by providing evolutionary and phylogenetic analysis for aquatic fish species. Since previous research determined that the functionality of cytochrome P450 is similar among multiple aquatic species we decided to compare the phylogenetic relationships between aquatic fish species that live in different ecological habitats and geographical regions. Multiple research studies have compared cytochrome P450 among aquatic, terrestrial, and microscopic organisms, but further research has been hinted in all studies for the need of evolutionary analysis for this specific enzyme. We believe that our results will enable us to determine any evolutionary process that may have arisen in cytochrome P450. Materials and Methods: We collected 13 completed cytochrome P450 1A mRNA sequences from GenBank. The Yellowhead Catfish was first examined for the sequence then BLAST was used to find other highly similar and more dissimilar sequences. Lengths of each sequence were between 13002700 base pairs long. The following sequences were obtained: Table 1: Species Accession Origin Length (BP) Author Yellowhead Catfish EF584508 Korea 2757 Kim,J.H., Raisuddin,S., Ki,J.S., Lee,J.S. and Han,K.N. Freshwater Minnow JN648712 Korea 1536 Kim,J.-H. Rainbow Trout AF059711 United States 2678 Carvan,M.J. III, Ponomareva,L.V., Solis,W.A., Matlib,R.S., Puga,A. and Nebert,D.W. Atlantic Salmon AF059711 Canada 1391 Leong,J., von Schalburg,K., Cooper,G., Moore,R., Holt,R.,Davidson,W.S. and Koop,B.F. Brook Trout AF539414 United States 1391 Rees,C.B. and Li,W. Zebrafish AF210727 United States 1530 Buhler,D.R. Lake Trout AF539415 United States 1391 Rees,C.B. and Li,W. Three-Spined S. HQ202281 Sweden 1387 Gao,K., Brandt,I., Goldstone,J.V. and Jonsson,M.E. Spotted Gar XM_006628888 United States 1414 Predicted Sequence: Bestplaced RefSeq; Gnomon Armoured Catfish HM043798 South America 1703 Parente,T.E.M., Rebelo,M.F., Woodin,B.R. and Stegeman,J.J. Cory Catfish HM043797 South America 2643 Parente,T.E.M., Rebelo,M.F., Woodin,B.R. and Stegeman,J.J. Turbot AJ310694 United Kingdom 1319 Craft, J. A. Yellow Croaker GQ281041 China 2598 Qian,Y., Qian,L. and Tong,L. Table 1 displays the species, accession number, origin, length, and author of the sequences gathered from GenBank. To examine this data ClustalW was then used to trim and align the sequences for the longest gaps. The program MEGA6.06 was then used to produce phylogenetic trees. Neighbor Joining and Maximum Parsimony trees were created with Kimura 2-parameter distance model. 1000 bootstrap replicas were also ran during the creation of the phylogenetic trees. To create each phylogenetic tree there is a need for the estimation of evolutionary distances using the pairwise distance method (Figure 1). This method compares the sequences of the species we decided to examine by calculating the percent difference between their nucleotides (Li). The Bootstrap method of phylogeny was used to determine trees that agree on an overall consensus of how the tree should be arranged. In other words, because the tree was run 1000 times (due to the 1000 replicas) the given tree is arranged due to reliability. Reliability is the probability that the members of a given clade are indeed member of that clade (Li). The Neighbor Joining method for phylogeny minimizes the overall branch length for a tree (Li). This method takes into account the varying rate of nucleotide substitution. One potential problem with this method is that the tree is subject to the accuracy of the sequences obtained due to potential statistical errors (Li). The Maximum Parsimony method for phylogeny calculates and finds a tree that requires the fewest number of changes. This is done by distinguishing between informative and uninformative sites, which gives us important information about taxa (Li). A site is only informative if there are only two different kinds of nucleotides present there. Kimura’s 2-parameter distance model was used as opposed to Jukes-Cantor distance model due to its recognition between transitional and transversional substitution rates (Li). Since transitions are more common than transversions it was decided that Kimura’s model would compensate for this bias. Also this decision was made due to the varying types of aquatic fish species present in our analysis. Results: From the data obtained from MEGA we observed consistent results of members within a clade for each tree that was created from the pairwise distance matrix (Figure 1). For each method used (Neighbor Joining and Maximum Parsimony) bootstrap trees were created as well (Figures 3 and 5 respectively). The bootstrap tree when compared to the Neighbor Joining tree did not differ from the parent tree in clade and member groupings, but only in branch lengths and organization to allow for better understanding. This is not the case for Maximum Parsimony. For the Kimura Neighbor Joining tree below (Figure 2) we observed a 100 percent agreement value of the freshwater minnow and the Zebrafish clade. Moving up the tree we observed a 96 percent agreement value for the clade containing the Armored Catfish, Cory Catfish, and Yellowhead Catfish. Within this clade there was a distinguishable sub-clade present that obtained a 98 percent agreement value for the Armored Catfish and Cory Catfish species. Above this clade a 99 percent agreement value was calculated for the clade containing Spotted Gar, Yellow Croaker, Turbot, Three-Spined Sickleback, Brook Trout, Atlantic Salmon, Lake Trout, and Rainbow Trout. Within this clade there were two 100 percent agreement values for the clades containing Turbot and Yellow Croaker as well as the clade containing Brook Trout, Lake Trout, Rainbow Trout, and Atlantic Salmon. As mentioned above, the bootstrap tree of phylogeny for the Neighbor Joining method (Figure 3) contained the exact same results as it’s parent tree. For the Maximum Parsimony tree below (Figure 4) we observed the Three-Spined Sickleback to be the most diverged from all other species because of its distinctively separate clade. Moving up the tree we observed a 100 percent agreement value for Turbot and Yellow Croaker clade. Above this clade we obtained a 63 percent agreement value for the remaining species clades and the Spotted Gar. Within this clade we observed three significant clades. The first one obtaining a 100 percent agreement value clade for the Atlantic Salmon, Brook Trout, Rainbow Trout, and Lake Trout. The second clade was found to have a 98 percent agreement value for the Freshwater Minnow and Zebrafish. The final clade had a 95 percent agreement value for Armored Catfish, Cory Catfish, and Yellowhead Catfish. Noticeable differences were observed in the bootstrap tree for Maximum Parsimony for the clade containing the Atlantic Salmon, Brook Trout, Rainbow Trout, and Lake Trout. This one clade from the Maximum Parsimony was made into two separate clades when calculated in bootstrap. Figure 1: Figure 1 contains the pairwise distance method for Kimura’s 2-parameter model of the species examined obtained from MEGA. Following each species is a description of their typical habitat (FWS = fresh water stream) and the geographical location the sample was obtained from. Figure 2: Figure 2 contains the Kimura’s 2-parameter model for the Neighbor Joining method of phylogeny. This tree was obtained from MEGA. Following each species is a description of their typical habitat (FWS = fresh water stream) and the geographical location the sample was obtained from. Figure 3: Figure 3 contains the Kimura 2-parameter model Neighbor Joining Bootstrap method of phylogeny. This free was obtained from MEGA. Following each species is a description of their typical habitat (FWS = fresh water stream) and the geographical location the sample was obtained from. Figure 4: Figure 4 contains the Maximum Parsimony method of phylogeny. This tree was obtained from MEGA. Following each species is a description of their typical habitat (FWS = fresh water stream) and the geographical location the sample was obtained from. Figure 5: Figure 5 contains the Maximum Parsimony Bootstrap method of phylogeny.This tree was obtained from MEGA. Following each species is a description of their typical habitat (FWS = fresh water stream) and the geographical location the sample was obtained from. Discussion: Through phylogenetic analysis we observed that the Rainbow Trout, Lake Trout, Brook Trout, and Atlantic Salmon are closely related. From the Neighbor Joining (Figure 2) method these species were consistently grouped together in the same clade for both the parent and bootstrap tree. However, a noticeable change occurred between Maximum Parsimony and its bootstrap tree. This method grouped these species in separate, distinct clades. This is an indication that these species are not closely related which disagrees with previous research that was conducted by Allendorf and Utter stating that these species were all apart of the Salmonidae family (Allendorf). The reasoning why we observed different results from each tree is due to the methods used in each process to make a tree (Li). We found this strange due to the fact that the Neighbor Joining method (and its bootstrap tree) and Maximum Parsimony method (just the parent tree) agreed on this clade with a 100 percent agreement value. The next clade we noticed consisted of the Freshwater Minnow and the Zebrafish. These species were also grouped consistently throughout each tree. Agreement values for Neighbor Joining and Maximum Parsimony for this clade were 100 and 98 percent, respectively. These species are known to a part of the Cyprinidae family. The last clade that contained the same family of fish was the clade with the Yellowhead Catfish, Armored Catfish, and Cory Catfish. There was a 96 percent agreement value in the Neighbor Joining tree and a 95 percent agreement value in the Maximum Parsimony tree. These species are known to be part of the Siluriforms family, which is consistent with our findings. We observed significant findings in the Turbot and the Yellow Croaker species, which consistently formed a clade together. The agreement value of their clades (in both methods) was found to be 100 percent. We believe that this finding is extraordinary due to the two species sharing no relation between each other except ecological habitat. Although future research is needed to confirm our assumptions, we believe that cytochrome P450 co-evolved within the two species. The remaining two species (Spotted Gar and Three-Spined Sickelback) we believe are classified as out-groups for this research. For all phylogenetic trees, these two species were consistently grouped in a branch of their own occasionally placed in relation to the clade containing the Turbot and Yellow Croaker. In conclusion, cytochrome P450 can be used as a biomarker. This is due to its important nature in metabolizing toxins and other foreign compounds. The findings obtained from this evolutionary and phylogenetic analysis can be used as a stepping-stone for future research regarding the determination of possible pollution events. This is due to inferences made from the geographical and ecological habitats of individual fish species and their groupings (phylogenetically) with other species. Research would involve monitoring of these waterways, and its species, to classify when cytochrome P450 diverged. References: Harskamp, James, Philip Britz-Mckibbin, and Joanna Y. Wilson. "Functional Screening of Cytochrome P450 Activity and Uncoupling by Capillary Electrophoresis." Analytical Chemistry 84.2 (2012): 862-866. Kloas, Werner, Caterina Wiedemann, Björn Hermelink, Thomas Behrends, Sven Würtz, Robert Opitz, Ralph Urbatzka, Ilka Lutz, Christoph Van Ballegooy, Achim Trubiroha, Constanze Pietsch, Nadja Neumann, Claudia Lorenz, Hana Kroupova, Oana Jagnytsch, and Frauke Hofmann. "Endocrine Disruption in Aquatic Vertebrates." Annals of the New York Academy of Sciences 1163.1 (2009): 187-200. Stegeman, J J, and P J Kloepper-Sams. "Cytochrome P-450 isozymes and monooxygenase activity in aquatic animals." Environmental Health Perspectives 71 (1987): 87-95. Allendorf, Fred W., and Fred M. Utter. “Gene duplication in the family Salmonidae:.” Hereditas 82.1 (1976): 19-24. Li, Wen, and Dan Graur. Fundamentals of molecular evolution. Sunderland, Mass.: Sinauer Associates, 1991.