Name: Discussion Section (circle one): D4 Bio 93 Discussion: Week
advertisement
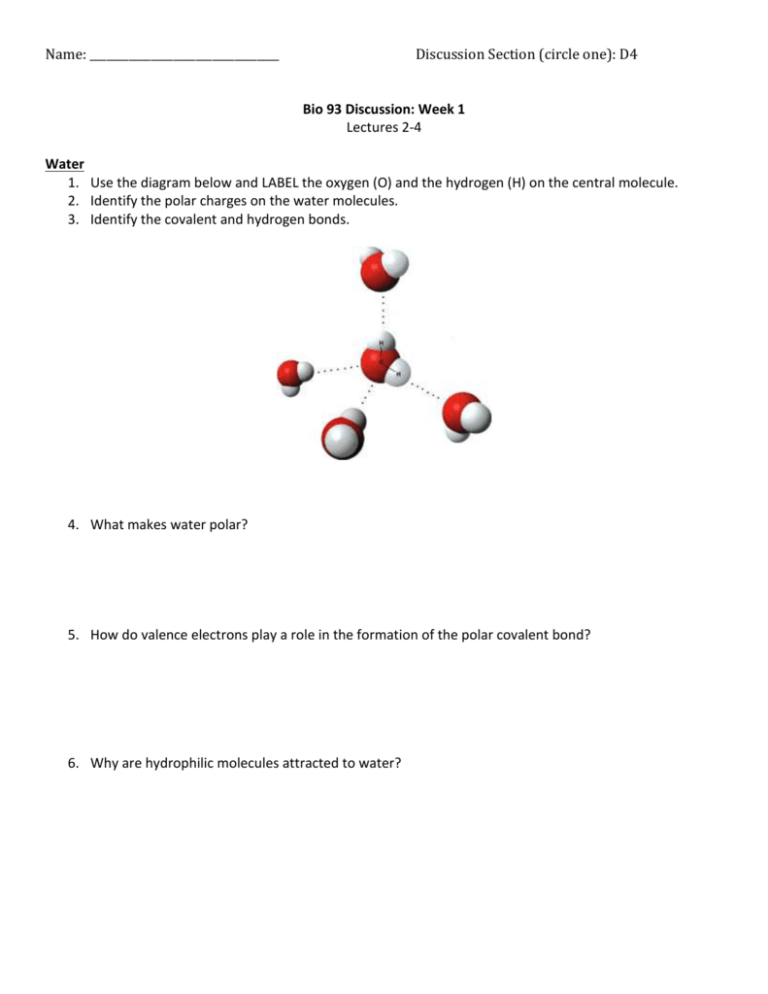
Name: __________________________________ Discussion Section (circle one): D4 Bio 93 Discussion: Week 1 Lectures 2-4 Water 1. Use the diagram below and LABEL the oxygen (O) and the hydrogen (H) on the central molecule. 2. Identify the polar charges on the water molecules. 3. Identify the covalent and hydrogen bonds. 4. What makes water polar? Oxygen is more electronegative than hydrogen. Given this, oxygen has a greater tendency to attract electrons (which is negative) TOWARDS itself, and AWAY from hydrogen atoms (due to atomic number and distance of valence electrons from nucleus). 5. How do valence electrons play a role in the formation of the polar covalent bond? A covalent bond is the sharing of a pair of valence electrons by two atoms. The shared electrons count as part of the atom’s valence shell. H has one spot to be filled hand O has two. 2 hydrogen atoms form a bond with 1 oxygen atom so that all valence shells are complete. 6. Why are hydrophilic molecules attracted to water? H2O, a charged polar molecule, surrounds other molecules to dissolve them. The negativity of the O and the positivity of the H atoms in the polar water molecule are attracted to opposite charges on molecules that are hydrophilic. Hydrophilicity/phobicity is dependent on charge. Acids/Bases/Buffers 7. Given this equation: H2PO4- HPO42- + H+ pKa = 7.2 At pH=7.2 you add some H+. Which way will your reaction push toward? Left or right? At pH=7.2 you add some OH-. Which way will your reaction push toward? Left or right? 8. Why are buffers important? What do they do? Buffers resist change in a pH of a solution. They donate H+ when the solution is depleted of H+. When a solution is in excess of H+ in the solution, they either donate OH- or accept H+. Carbon-Based Macromolecules 9. Polymerization reactions are also called _________________ or ___________________. 10. Depolymerization reactions are also called _______________________. 11. Draw a simple saturated and unsaturated fatty acid. Which ones can be densely packed? Saturated Which one helps make the membranes fluid? How? Unsaturated fats. Unsaturated fats have double bonds, forming “kinks.” These kinks cause the membrane to be more fluid 12. Why are saturated fats generally worse for health than unsaturated fats? They are less soluble and less fluid than unsaturated fats, making them more likely to clog blood vessels. 13. Name 4 macromolecules. Which macromolecule is used for cellular respiration? Nucleic acids, carbohydrates, lipids and proteins; carbohydrates are used for cellular respiration 14. Why are hydrogen bonds important in biology? 2 15. Match the Following types of bond with their correct pair Peptide Bond Ester linkage Phosphodiester linkage Glycosidic linkage Connects two sugars Connects two amino acids Connects glycerol with fatty acid Connects polynucleotides Functional Groups: 16. A. B. C. Answer the following questions: How many R groups are present? Circle the R groups that are nonpolar. How many peptide bonds are present? 3