Old Exam

CHE 230
Exam #3 Spring 2011 Take Home Portion
Name _______________________
Please initial each of the statements below to indicate that you understand the instructions.
This exam is independent work; I am not to discuss or work with anyone on the exam. _______
This is a closed book/note exam; I am only to use the provided equation sheet and periodic table. ______
This portion of the exam is to be completed in one hour or less and in one sitting; once I look at the exam, I only have one hour to work on it. _______
Start date/time: ___________________
C. Lab Problems/Short Answer. Calculations will be required to answer these questions. Make sure to show work for partial credit and present answers with the correct number of significant digits. In many cases, the numerical result is not a sufficient answer. Supporting text will be required to interpret the result.
Problem #1. Refer to the Merck Index entry for fervenulin to the right to answer the following questions.
Which wavelength is most sensitive for the ultraviolet spectroscopic analysis of fervenulin?
Explain.
Which recommended wavelength is closest to the visible region? Calculate the frequency of electromagnetic radiation associated with this wavelength.
UVVIS spectroscopy is typically performed at “intermediate” absorbances. Using information from Beer’s
Law, describe how you would prepare a standard of fervenulin to target an intermediate absorbance of approximately 0.1.
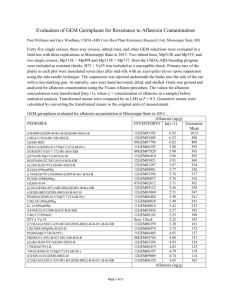
Problem #2. Refer to the attached chromatographs AND the provided quantitative chromatographic information for a caffeine analysis (similar to what we had done in lab) to answer the following questions.
Which peak is the caffeine peak? How do you know?
Calculate the resolution (as best you can) for peaks #5 and #6 in the energy drink sample. Do you consider this “good” resolution? Why/why not?
Create a calibration curve for caffeine on the grid below based on the chromatographic results.
Estimate (show work) the concentration of caffeine in the prepared energy drink sample based on your calibration curve.
Why is it difficult to estimate the caffeine concentration with these particular results? From a methodology perspective, how could you improve this?
Problem #3. Aflatoxin is an extremely toxic chemical produced by a rare fungus occasionally found in food. It can be analyzed using luminescence spectroscopy. A calibration curve for aflatoxin in cyclohexane is provided. Using the calibration curve and sample preparation steps described, calculate the concentration of aflatoxin as directed.
For the analysis of aflatoxin on corn meal, 53.0 g of corn meal was heated in the presence of 50.0 mL of
10% acetic acid to hydrolyze the aflatoxin. Following hydrolysis, the aqueous hydrolysate was extracted two times with 20.0 mL of cyclohexane. The two extracts were combined for a total volume of 40.0 mL. A
2.0 mL aliquot of the combined extracts was placed into a fluorescence cuvette and analyzed. The resulting fluorescence intensity was 227. o Calculate the concentration of aflatoxin (in ng/mL) in the prepared corn meal sample. o Use the sample preparation steps to back-track the aflatoxin concentration (in ppb or ng/g) in the original corn meal sample.
o According to the FDA, alflatoxin should not exceed 20 ppb in food samples. Does this sample exceed the legal limit?
Aflatoxin was recently synthesized in a pharmaceutical lab. 0.105 g of unpurified product was diluted to
100.0 mL in cyclohexane. 1.00 mL of the dissolved sampled was then further diluted to 1000.0 mL in cyclohexane. A 2.0 mL aliquot of this sample was placed into a fluorescence cuvette and analyzed. The resulting fluorescence intensity was 803. o Calculate the concentration of aflatoxin (in ng/mL) in the prepared sample. o Use the sample preparation steps to back-track the mass (mg) of aflatoxin in the synthesized sample. o Calculate the percent purity of aflatoxin in the synthesized sample. Is this sample very pure?
Explain.
End time: ___________________
My signature below indicates that I have adhered to all the rules associated with this take-home exam. I understand that any academic integrity violations will be reported to the Vice President of Academic
Affairs.______________________________________