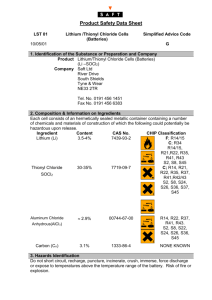
Word EquationàSkeleton EquationàBalanced Equation
advertisement

Word EquationSkeleton EquationBalanced Equation Name______________________ Date_______________Pd_____ 1.) Carbon tetrahydride gas is reacted with oxygen to produce carbon dioxide gas and water vapor. 2.) Aqueous calcium chloride is combined with aqueous lithium phosphate to form solid calcium phosphate and aqueous lithium chloride. 3.) Solid carbon is burned with oxygen to produce carbon monoxide gas. 4.) When heated, solid magnesium carbonate produces a white solid magnesium oxide and releases carbon dioxide gas. Word EquationSkeleton EquationBalanced Equation 5.) Lithium reacts with oxygen to form solid lithium oxide. 6.) Chlorine reacts with potassium iodide to form solid potassium chloride an releases iodine gas. 7.) Mercury (II) nitrate solution reacts with potassium iodide solution to give a mercury (II) iodide precipitate and potassium nitrate solution. 8.) Water and dinitrogen trioxide produce nitrous acid. Word EquationSkeleton EquationBalanced Equation Name______________________ Date_______________Pd_____ 1.) Carbon tetrahydride gas is reacted with oxygen to produce carbon dioxide gas and water vapor. 2.) Aqueous calcium chloride is combined with aqueous lithium phosphate to form solid calcium phosphate and aqueous lithium chloride. 3.) Solid carbon is burned with oxygen to produce carbon monoxide gas. 4.) When heated, solid magnesium carbonate produces a white solid magnesium oxide and releases carbon dioxide gas. Word EquationSkeleton EquationBalanced Equation 5.) Lithium reacts with oxygen to form solid lithium oxide. 6.) Chlorine reacts with potassium iodide to form solid potassium chloride an releases iodine gas. 7.) Mercury (II) nitrate solution reacts with potassium iodide solution to give a mercury (II) iodide precipitate and potassium nitrate solution. 8.) Water and dinitrogen trioxide produce nitrous acid.