Chapter 8 & 9 Outline
advertisement

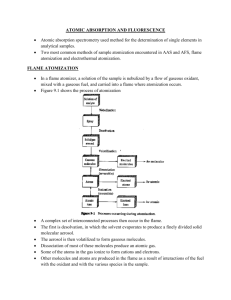
ATOMIC ABSORPTION AND FLUORESCENCE Atomic absorption spectrometry used method for the determination of single elements in analytical samples. Two most common methods of sample atomization encountered in AAS and AFS, flame atomization and electrothermal atomization. FLAME ATOMIZATION In a flame atomizer, a solution of the sample is nebulized by a flow of gaseous oxidant, mixed with a gaseous fuel, and carried into a flame where atomization occurs. Figure 9.1 shows the process of atomization A complex set of interconnected processes then occur in the flame. The first is desolvation, in which the solvent evaporates to produce a finely divided solid molecular aerosol. The aerosol is then volatilized to form gaseous molecules. Dissociation of most of these molecules produce an atomic gas. Some of the atoms in the gas ionize to form cations and electrons. Other molecules and atoms are produced in the flame as a result of interactions of the fuel with the oxidant and with the various species in the sample. A fraction of the molecules, atoms, and ions are also excited by the heat of the flame to yield atomic, ionic, and molecular emission spectra. TYPES OF FLAMES The burning velocities listed in the fourth column of table 9-1 are important because flames are stable only in certain ranges of gas flow rates. If the gas flow rate does not exceed the burning velocity, the flame propagates back into the burner, giving flashback. As the flow rate increases, the flame rises until it reaches a point above the burner where the flow velocity and the burning velocity are equal. This region is where the flame is stable. At higher flow rates, the flame rises and eventually reaches a point where it blows off the burner. So important to control the flow rate of the fuel-oxidant mixture. This flow rate very much depends on the type of fuel and oxidant being used FLAME STRUCTURE Important regions of a flame include the primary combustion zone, the interzonal region, and the secondary combustion zone, as shown in Figure 9-2. Relative size of these regions vary considerably with the fuel-to-oxidant ratio as well as with the type of fuel and oxidant. Free atoms are prevalent in the interzonal region, it is the most widely used part of the flame for spectroscopy. In the secondary reaction zone, the products of the inner core are converted to stable molecular oxides that are then dispersed into the surrounding. A flame profile provides useful information about the processes that go on in different parts of a flame; it is a contour plot that reveals regions of the flame that have similar values for a variable of interest. Variables include temperature, chemical composition, absorbance, and radiant or fluorescence intensity. FLAME ABSORPTION PROFILES Figure 9-4 shows typical absorption profiles for three elements. The initial increase in absorbance as the distance from the base increases results from an increased number of magnesium atoms produced by the longer exposure to the heat of the flame. As the secondary combustion zone is approached, however, appreciable oxidation of the magnesium begins. This process eventually leads to a decrease in absorbance because the oxide particles formed do not absorb at the observation wavelength. To achieve maximum analytical sensitivity, then, the flame must be adjusted up and down with respect to the beam until the region of maximum absorbance is located. Silver, as shown in figure 9-4, a continuous increase in the number of atoms, and thus the absorbance, is observed from the base to the periphery of the flame. Chromium, which form very stable oxides, shows a continuous decrease in absorbance beginning close to the burner tip; this observation suggests that oxide formation predominates from the start. These observations suggest that a different portion of the flame should be used for the determination of each of these elements. FLAME ATOMIZERS Flame atomizers are used for atomic absorption, fluorescence, and emission spectroscopy. The aerosol, formed by the flow of oxidant, is mixed with fuel and passes a series of baffles that remove all but the finest solution droplets. The baffles cause most of the sample to collect in the bottom of the mixing chamber where it drains to a waste container. The aerosol, oxidant, and fuel are then burned in a slotted burner to provide a 5- to 10cm high flame. Laminar-flow burners produce a relatively quiet flame and a long path length for maximizing absorption. These properties tend to lead to enhanced sensitivity and reproducibility to AAS. FUEL AND OXIDANT REGULATORS An important variable that requires close control in flame spectroscopy is the flow rate of both oxidant and fuel. It is desirable to be able to vary each over a broad range so that optimal atomization conditions can be determined experimentally. Fuel and oxidant are usually combined in approximately stoichiometric amounts. For the determination of metals, flame that contains an excess of fuel is often desirable. Flow rates are usually controlled by means of double-diaphragm pressure regulators followed by needle valves in measuring flow rates is the rotameter. PERFORMANCE CHARACTERISTICS OF FLAME ATOMIZERS Most reproducible of all liquid-sample-introduction methods. There are two primary reasons for the lower sampling efficiency of the flame. First, a large portion of the sample flows down the drain. Second, the residence time of individual atoms in the optical path in the flame is brief. ELECTROTHERMAL ATOMIZATION Generally provide enhanced sensitivity because the entire sample is atomized in a short period, and the average residence time of the atoms in the optical path is a second or more. They are used for vaporizing samples in inductively coupled plasma emission spectroscopy. In electrothermal atomizers, a few microliters of sample is first evaporated at a low temperature and then ashed at a somewhat higher temperature in an electrically heated graphite tube similar to the one in figure 9-6. After ashing, the current is rapidly increased to several hundred amperes, which causes the temperature to rise to 2000 degrees Celsius to 3000 degrees Celsius; atomization of the sample occurs in a period of a few milliseconds to seconds. The absorption of fluorescence of the atomic vapor is then measured in the region immediately above the heated surface. ELECTROTHERMAL ATOMIZERS Atomization occurs in a cylindrical graphite tube that is open at both ends and that has a central hole for introduction of sample by means of a micropipette. Figure 9-6a illustrates the so-called L’vov platform, which is often used in graphite furnaces. The sample is evaporated and ashed on this platform. When the tube temperature is increased rapidly, however, atomization is delayed because the sample is no longer directly on the furnace wall. As a result, atomization occurs in an environment in which the temperature is not changing so rapidly, which improves the reproducibility of analytical signals. Experiments show that reducing the natural porosity of the graphite tube minimizes some sample matrix effects and poor reproducibility associated with graphite furnace atomization. OUTPUT SIGNALS At a wavelength at which absorbance or fluorescence occurs, the transducer output rises to a maximum after a few seconds of ignition followed by a rapid decay back to zero as the atomization products escape into the surroundings. Quantitative determinations are usually based on peak height, although peak area is also used. Typical output signals are shown on Figure 9-7. PERFORMANCE CHARACTERISTICS OF ELECTROTHERMAL ATOMIZERS Advantage of unusually high sensitivity for small volumes between 0.5 and 10 µL are used. The relative precision of electrothermal methods is generally in the range of 5% to 10%. Because of the heating-cooling cycles, furnace methods are slow, typically requiring several minutes per element. Disadvantage is that the analytical range is relatively narrow. ANALYSIS OF SOLIDS WITH ELECTROTHERMAL ATOMIZERS Most methods, samples are introduced as solutions. Several reports, however, have described the use of this type of atomizer for the direct for the direct analysis of solid samples. SPECIALIZED ATOMIZATION TECHNIQUES Most common sample-introduction and atomization techniques are flames or electrothermal vaporizers. GLOW-DISCHARGE ATOMIZATION A glow-discharge device produces an atomized vapor that can be swept into a cell for absorption measurements. Figure 9-8a shows a glow-discharge cell. The sample is pressed against this hole with a torque screw so that it seals the tube. Six fine streams of argon gas from tiny nozzles arranged in a circular pattern above the sample impinge on the sample surface in a hexagonal pattern. The argon is ionized by a current between an anode supporting the nozzles and the sample, which acts as a cathode. As a result of sputtering, six craters quickly form on the sample surface as shown on figure 98b. The sputtered atoms are drawn by a vacuum to the axis of the cell where they absorb radiation from the spectrometer source. The sample must be an electrical conductor or must be made into a pellet with a powdered conductor such as finely ground graphite or copper. HYDRIDE ATOMIZATION Atomization of the hydrides requires only that they be heated in a quartz tube, as shown in figure 9-9. COLD-VAPOR ATOMIZATION The cold-vapor technique is an atomization method applicable only to the determination of mercury because it is the only metallic element that has as appreciable vapor at ambient temperature. Vital importance currently because of the toxicity of organic mercury compounds and their widespread distribution in the environment. One popular method for this determination is cold vaporization followed by atomic absorption spectrophotometry. ATOMIC ABSORPTION INSTRUMENTATION Instruments consist of a radiation source, a sample holder, a wavelength selector, a detector, and a signal processor and readout. Sample holder is the atomizer cell that contains the gaseous atomized sample. RADIATION SOURCES Are potentially highly specific because atomic absorption lines are remarkably narrow and because electronic transition energies are unique for each element. Narrow line widths create a problem. The problem created by the limited width of atomic absorption lines has been solved by the use of line sources with bandwidths even narrower than the absorption line width. HOLLOW-CATHODE LAMPS The most common source for atomic absorption measurements is the hollow-cathode lamp, shown in Figure 9-11. It consists of a tungsten anode and a cylindrical cathode sealed in a glass tube filled with neon or argon at a pressure of 1 to 5 torr. Ionization of the inert gas occurs when a potential difference on the order of 300 V is applied across the electrodes, which generates a current of about 5 to 15mA as ions and electrons migrate to the electrodes. If the voltage is sufficiently large, the gaseous cations acquire enough kinetic energy to dislodge some of the metal atoms from the cathode surface and produce an atomic cloud in a process called sputtering. A portion of the sputtered metal atoms are in excited states and thus emit their characteristics radiation as the return to the ground state. Eventually, the metal atoms diffuse back to the cathode surface or to the glass walls of the tube and are redeposited. The cylindrical configuration of the cathode tends to concentrate the radiation in a limited region of the metal tube; this design also enhances the probability that redeposition will occur at the cathode rather than on the glass walls. Efficiency depends on its geometry and the operating voltage. High voltages, and thus high currents, lead to greater intensities. This advantage is offset somewhat by an increase in Doppler broadening of the emission lines from the lamp. The greater currents produce an increased number of unexcited atoms in the cloud. Unexcited atoms, in turn, are capable of absorbing the radiation emitted by the excited ones. This self-absorption leads to lowered intensities, particularly at the center of the emission band. ELECTRODLESS DISCHARGE LAMPS Electrodless discharge lamps (EDLs) are useful sources of atomic line spectra and provide radiant intensities usually one to two orders of magnitude greater than hollowcathode lamps. Constructed from a sealed quartz tube containing a few torr of an inert gas such as argon and a small quantity of the metal (or its salt) whose spectrum is of interest. Contains no electrode but instead is energized by an intense field of radio-frequency or microwave radiation. Ionization of the argon occurs to give ions that are accelerated by the high-frequency component of the field until they gain sufficient energy to excite the atoms of the metal whose spectrum is sought. Their performance is not as reliable as that of the hollow-cathode lamp, but for elements such as Se, As, Cd and Sb, EDLS exhibit better detection limits than do hollow-cathode lamps. SOURCE MODULATION Necessary to eliminate interferences caused by emission of radiation by the flame. Much of this emitted radiation is, removed by the monochromator. Emitted radiation corresponding in wavelength to the monochromator setting is inevitably present in the flame because of excitation and emission of analyte atoms and flame gas species. To eliminate, it is necessary to modulate the output of the source so that its intensity fluctuates at a constant frequency. The detector then receives two types of signal, an alternating one from the source and a continuous one from the flame. These signals are converted to the corresponding types of electrical response. A simple high-pass RC filter (section 2.2.5) can then be used to remove the unmodulated dc signal and pass the ac signal for amplification. A simple and entirely satisfactory way of modulating the emission for the source is to interpose a circular metal disk, or chopper, in the beam between the source and the flame. Other types of electromechanical modulators including tuning forks with vanes attached to alternately block and transmit the beam (figure 5-7c) and devices that rotate a vane through a fixed arc to perform the same function. SINGEL-BEAM INSTRUMENTS Consists of several hollow-cathode sources, a chopper or a pulsed power supply, an atomizer, and a simple grating spectrophotometer with a photomultiplier transducer. DOUBLE-BEAM INSTRUMENTS Figure 9-13 The beam from the hollow-cathode source is split by a mirrored chopper, one half passing through the flame and the other half around it. The two beams are then recombined by a half-silvered mirror and passed into a CzernyTurner grating monochromator; a photomultiplier tube serves as the transducer. The output from the latter is the input to a lock-in amplifier that is synchronized with the chopper drive. The ratio between the reference and sample signal is then amplified and fed to the readout, which may be a digital meter or a computer. It should be noted that the reference beam in atomic double-beam instruments does not pass through the flame and thus does not correct for loss of radiant power due to absorption or scattering by the flame itself. INTERFERENCES IN ATOMIC ABSORPTION SPECTOSCOPY Interferences of two types are encountered. Spectral interferences arise when the absorption or emission of an interfering species either overlaps or lies so close to the analyte absorption or emission that resolution by the monochromator becomes impossible. Chemical interferences result from various chemical processes occurring during atomization that alter the absorption characteristics of the analyte. SPECTRAL INTERFERENCES Because the emission lines of hollow-cathode sources are so very narrow, interference because of overlapping lines is rare. They also result from the presence of combustion products that exhibit broadband absorption or particulate products that scatter radiation. Both reduce the power of the transmitted beam and lead to positive analytical errors. A much more troublesome problem occurs when the source of absorption of scattering originates in the sample matrix Spectral interference because of scattering by products of atomization is most often encountered when concentrated solutions containing elements Ti, Zr, and W- which form refractory oxides-are aspirated into the flame. Interference caused by scattering may also be a problem when the sample cantains organic species or leaves carbonaceous particles that are capable of scattering light. With flame atomization, spectral interferences by matrix products are not widely encountered and often can be avoided by variations in the analytical variables, such as flame temperature and fuel-to-oxidant ratio. If the source of interference is known, an excess of the interfering substance can be added to both sample and standards. TWO-LINE CORRECTION METHOD The two-line correction procedure uses a line from the source as a reference. This line should lie as close as possible to the analyte line but must not be absorbed by the analyte. The reference line may be from an impurity in the hollow-cathode, a neon or argon line from the gas contained in the lamp, or nonresonant emission line of the element that is being determind. BACKGROUND CORRECTION BASED ON THE ZEEMAN EFFECT Figure 9-15 shows an instrument which provides a background correction based on the Zeeman Effect. When an atomic vapor is exposed to a strong magnetic field (~10kG), a splitting of electronic energy levels of the atoms takes place that leads to formation of several absorption lines for each electronic transition. These lines are separated from one another by about 0.01nm, with the sum of the absorbances for the lines being exactly equal to that of the original line from which they were formed. This is the ZEEMAN EFFECT, Zeeman effect instruments provide a more accurate correction for background than the methods described earlier. These instruments are useful for electrothermal atomizers and permit the direct determination of elements in samples such as urine and blood CHEMICAL INTERFERENCES Chemical interferences are more common than spectral interferences. FORMATION OF COMPOUNDS OF LAW VOLATILITY The most common type of interference is by anions that form compounds of low volatility with the analyte and thus reduce the fraction of the analyte that is atomized. Low results are the outcome. Cation interference has been observed as well. Interferences caused by formation of species of low volatility can often be eliminated or moderated by use of higher temperatures. Releasing agents, which are cations that react preferentially with the interferant and prevent its interaction with the analyte, can be used. Protective agents prevent interference by forming stable but volatile species with analyte. SAMPLE PREPARATION A disadvantage of flame spectroscopic methods is the requirement that the sample be introduced into the excitation source in the form of a solution, most commonly an aqueous one. Many materials of interest, such as soils, animal tissues, plants, petroleum products, and minerals not directly soluble in common solvents, and extensive preliminary treatment is often required to obtain a solution of the analyte in a form ready for atomization. Decomposition of materials require rigorous treatment of the sample at high temperatures accompanied by a risk of losing the analyte by volatilization or as particulates in smoke. The reagents used in decomposing a sample often introduce the kinds of chemical and spectral interferences mentioned. The analyte may be present in these reagents as an impurity. It is not uncommon in trace analyses to find that reagents are a large source of the analyte than the samples. Some of the common methods used for decomposing and dissolving samples include treatment with hot mineral acids, oxidation with liquid reagents, such as sulfuric, nitric, or perchloric acids (wet ashing); combustion in an oxygen bomb or other closed container to avoid loss of analyte; ashing at a high temperature; and high-temperature fusion with reagents such as boric oxide, sodium carbonate, sodium peroxide, or potassium pyrosulfate. One of the advantages of the electrothermal atomization is that some materials can be atomized directly, thus avoiding the solution step. SAMPLE INTRODUCTION BY FLOW INJECTION FIA methodology serves as an excellent means of introducing samples into a flame atomic absorption. ORGANIC SOLVENTS Enhanced absorbances could be obtained if the solutions contained low-molecular-weight alcohols, esters, or ketones. The effect of organic solvents is largely attributed to increased nebulizer efficiency; the lower surface tension of such solutions results in smaller drop sizes and a resulting increase in the amount of sample that reaches the flame. The more rapid solvent evaporation may also contribute to the effect. CALIBRATION CURVES Atomic absorption should follow Beers Law with absorbance being directly proportional to concentration Calibration curves are often nonlinear APPLICATIONS OF AAS AAS is a sensitive means for the quantitative determination of more than sixty metals or metalloid elements. ATOMIC FLUORESCENCE SPECRTOSCOPY Atomic fluorescence spectroscopy provides a useful and convenient means for the quantitative determination of a reasonably large number of elements. INSTRUMENTATION The sample container is most commonly a flame but may also be an electrothermal atomization cell, a glow discharge, or an inductively coupled plasma. SOURCES A continuum source would be desirable for atomic fluorescence measurements. Unfortunately, however , the output power of most continuum sources over a region as narrow as an atomic absorption line is too low to provide sufficient sensitivity for atomic fluorescence. Perhaps the most widely used sources for atomic fluorescence have been EDLs. Unfortunately, this type of lamp is not available for many elements. Lasers, with their high intensities and narrow bandwidths, would appear to be the ideal source for atomic fluorescence measurements. DISPERSIVE INSTRUMENTS They consists of a modulated source, an atomizer (flame or non-flame), a monochromator or an interference filter system, a detector, and a signal processor and readout. NONDISPERSIVE INSTRUMENTS No monochromator or filter should be necessary for atomic fluorescence measurements when an EDL or hollow-cathode lamp serves as the excitation source because the emitted radiation is, in principle, that of a single element and will thus excite only atoms of that element. INTERFERENCES Interferences encountered are generally of the same type and of about the same magnitude as those found in atomic absorption spectroscopy. APPLICATIONS Atomic fluorescence methods have been applied to the determination of metals is such materials as lubricating oils, seawater, geological samples, metallurgical samples, environmental samples, and agricultural samples.