1. a) 25% b)86% 2. For my opinion, I think the way to make
advertisement

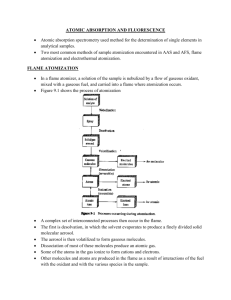
1. a) 25% b)86% 2. For my opinion, I think the way to make correction for background absorption caused by sample matrices is require storage of a reference spectrum from a biomembrane before the interaction with a substrate. Alteration of this membrane caused by external influence are then stored in a sequence of single channel spectra which are then converted into a transmittance or absorbance spectra by means of the stored reference spectrum. 3. Single beam UV-Vis Spectrometer It is used to determine the absorption light from a sample and it can be used as a detector of HPLC. The concentration of analyst in solution can be determined by measuring the absorbance of single wavelength and applying the Beer-Lambert Law. First, place the sample in the Uv-Vis beam and record the absorbance versus wavelength. Then, the single beam spectrometer utilizes one beam of light that oases through the sample and the intensity of the light reflected from a reference is measured without the sample. 4. Flame atomization absorption spectrometry (FAAS) It is a common technique to detect the metals and metalloids in environmental samples. It can be used to measure the solutions at the parts per million levels which equivalent to one gram of element per 100kg of solution. It is suitable to be used for wide range of analysis. This technique is based on the fact that ground state metals absorb light at specific wavelengths. Metal ions of a solutions are converted to atomic state by means of a flame. Light of appropriate wavelength is supplied and the amount of light absorbed can be measured against a standard curve. The stages of sample preparation / preconcentration, nebulization and atomization can be used to improve the sensitivity of the technique. The sample preparation stage is the simplest methods to improve the sensitivity of the technique by increasing the concentration of the sample solution. The nebulization method is the physical process of changing the bulk solution into a spray of fine droplets and mixing the droplets with the combustion gases. The atomization method is the physical process changes occurring to the solution aerosol in a flame. The basic principal of this technique is requiring a liquid sample to be aspirated, aerosolized and mixed with combustible gases. The mixture is ignited in a flame whose temperature ranges from 2100 to 2800℃. During combustion, atoms of the element of interest in the sample are reduced to free, unexcited ground state atoms which absorb light at characteristic wavelengths. 5. Graphite furnace atomization absorption spectrometry (GAAS) The technique is used to The technique is used to perform the quantitative analysis of metals in a wide variety of samples. It uses a graphite-coated furnace to vaporize the sample. Briefly, the technique is based on the fact that free atoms will absorb light at frequencies or wavelengths characteristic of the element of interest (hence the name atomic absorption spectrometry). Within certain limits, the amount of light absorbed can be linearly correlated to the concentration of analyte present. Free atoms of most elements can be produced from samples by the application of high temperatures. In GFAAS, samples are deposited in a small graphite or pyrolytic carbon coated graphite tube, which can then be heated to vaporize and atomize the analyte. The atoms absorb ultraviolet or visible light and make transitions to higher electronic energy levels. Applying the Beer-Lambert law directly in AA spectroscopy is difficult due to variations in the atomization efficiency from the sample matrix, and nonuniformity of concentration and path length of analyte atoms (in graphite furnace AA). Concentration measurements are usually determined from a working curve after calibrating the instrument with standards of known concentration. 6. Application Flame atomization absorption spectrometry (FAAS) Low to accuracy determine of mercury as the limited atom number density in the light path associated with poor nebulization and atomization. 0.2 mg L–1 at 253.7 nm graphite furnace atomization absorption spectrometry (GAAS) Can be extremely low Applicable for relatively clean samples Achieved by chemical vapor generation Reviewed for the determination of As, Bi, Ge, In, Pb, Sb, Se, Sn, Te and Tl (as well as Hg). 0.0598 mg L–1 for a 2 min Cloud point methodology Carbon Rod Atomizer 63