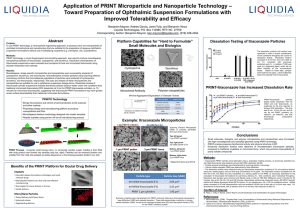
Supplementary material Supplementary Table 1. Schematic
advertisement

Supplementary material Supplementary Table 1. Schematic representation of one phase in a three-phase crossover study. Washout time between phases was six weeks. Day Pretreatment with antimycotic at 07:00 a.m. Tramadol at 08:00 a.m. 1 Placebo, terbinafine 250mg or itraconazole 200mg 2 Placebo, terbinafine 250mg or itraconazole 200mg 3 Placebo, terbinafine 250mg or itraconazole 200mg 4 Placebo, terbinafine 250mg or itraconazole 200mg 5 Placebo, terbinafine 250mg or itraconazole 200mg Tramadol 50mg Supplementary text - Bioanalytical methods The concentrations of plasma tramadol and M1 were measured on an API 3000 liquid chromatography–tandem mass spectrometry system (AB Sciex, Toronto, ON, Canada) as previously described [1], with minor modifications. Prior to analysis a simple protein precipitation of plasma samples with acetonitrile was performed, excluding the samples containing very low plasma drug concentrations (48-h samples), which were prepared by use of a MCX solid phase extraction (Waters, Milford, MA). Chromatography was performed on an Atlantis T3 analytical column (2.1x100 mm; Waters) using 10mM formic acid with 0.1% (v/v) trifluoroacetic acid (channel A) and methanol (channel B) as the mobile phase. Tramadol-d6 and O-desmethyltramadol- d6 served as internal standards and were added to plasma samples, plasma quality control samples and plasma reference standards before other pre-analytical procedures. The recovery for both analytes were > 75%. The mass spectrometer was operated in positive multi-reaction monitoring (MRM+) detection mode with electro spray ionization. The selected ion transitions used for quantification were: m/z 264 to m/z 58 for tramadol, m/z 250 to m/z 58 for M1, and m/z 270 to m/z 64 and m/z 256 to m/z 64 for internal standards, respectively. The limit of quantification for plasma tramadol and M1 was 0.02 ng/ml. The interday coefficients of variation (CV%) were for tramadol 7.2% at 2 ng/ml, 7.8% at 10 ng/ml and 4.6% at 100 ng/ml, and for M1 4.9% at 1.75 ng/ml, 8.5% at 8.75 ng/ml, and 5.4% at 87.5 ng/ml. The interday accuracy, expressed as the percentage relative error (RE%) from nominal concentrations (2, 10, 100 mg/ml) was < 10%. Plasma concentrations of terbinafine, itraconazole and hydroxyitraconazole were determined as described before [2,3]. Their limit of quantification was 10 ng/ml. The CV for terbinafine was 2.5% at 21 ng/ml, 3.7% at 92 ng/ml, and 2.0% at 699 ng/ml. The CV for itraconazole was 8.0% at 19 ng/ml, 1.0% at 192 ng/ml, and 1.7% at 1200 ng/ml. The CV for hydroxyitraconazole was 3.5% at 19 ng/ml, 0.9% at 192 ng/ml, and 2.2% at 1200 ng/ml. Similarly, concentrations of 5-HT and 5-HIAA in whole blood were analyzed as described earlier [4]. Limits of quantification were 30 nmol/l for 5-HT and 3 nmol/l for 5HIAA. The CVs of both analytes were <10% at these levels and <5% in the actual concentration ranges encountered in the study. Genotyping for CYP2D6 was performed using a two-step multiplex primer extension method [5]. The concentrations of plasma tramadol, O-desmethyltramadol (M1), terbinafine, itraconazole and hydroxyitraconazole were determined in an in house lab, i.e. in the laboratory of the Department of Clinical Pharmacology, University of Helsinki. References 1. Patel BN, Sharma N, Sanyal M, Shrivastav PS (2009) An accurate, rapid and sensitive determination of tramadol and its active metabolite O-desmethyltramadol in human plasma by LC-MS/MS. J Pharm Biomed Anal 49 (2):354-366. doi:10.1016/j.jpba.2008.10.030 2. Kovarik JM, Kirkesseli S, Humbert H, Grass P, Kutz K (1992) Dose-proportional pharmacokinetics of terbinafine and its N-demethylated metabolite in healthy volunteers. The British journal of dermatology 126 Suppl 39:8-13 3. Allenmark S, Edebo A, Lindgren K (1990) Determination of itraconazole in serum with high-performance liquid chromatography and fluorescence detection. Journal of chromatography 532 (1):203-206 4. Saarikoski T, Saari TI, Hagelberg NM, Neuvonen M, Neuvonen PJ, Scheinin M, Olkkola KT, Laine K (2013) Rifampicin markedly decreases the exposure to oral and intravenous tramadol. Eur J Clin Pharmacol 69 (6):1293-1301. doi:10.1007/s00228-012-1460-x 5. Sistonen J, Fuselli S, Levo A, Sajantila A (2005) CYP2D6 genotyping by a multiplex primer extension reaction. Clin Chem 51 (7):1291-1295. doi:10.1373/clinchem.2004.046466