Centennial Park Water Testing Lab
advertisement

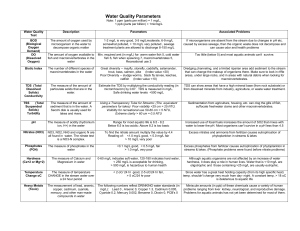
Centennial Park Water Testing Lab - Additional Info Preferred ranges pH The pH of a sample of water is a measure of the concentration of hydrogen ions. At a higher pH, there are fewer free hydrogen ions, and a change of one pH unit reflects a tenfold change in the concentration of the hydrogen ion. For example, there are 10 times as many hydrogen ions available at a pH of 7 than a pH of 8. The pH scale ranges from 0 to 14. A pH of 7 is considered to be neutral. Substances with pH less than 7 are acidic; substances with pH greater than 7 are basic. The pH of water determines the solubility (amount of soluble minerals and nutrients that can be dissolved into the water) and biological availability (amount of minerals and nutrients that can be utilized by aquatic life). For example, in addition to affecting how much and what form of phosphorus is most abundant in the water, pH also determines whether aquatic life can use it. In the case of heavy metals, the degree to which they are soluble determines their toxicity. Metals tend to be more toxic at lower pH because they are more soluble, if they are more soluble then they tend to be dissolved into the water. If they dissolved into the water then they are more likely to be ingested by aquatic organisms. At higher pH’s they will drop to the bottom of a stream and become part of the sediment. Photosynthesis (in submerged aquatic plants and algae) uses up hydrogen molecules, which causes the concentration of hydrogen ions to decrease and therefore the pH to increase. For this reason, pH may be higher during daylight hours and during the growing season, when photosynthesis is at a maximum. Respiration and decomposition processes lower pH. Rotting grass and leaves in a lake or stream might result in low pH values. Fortunately, lake or stream water is complex; it is full of chemical "shock absorbers" that prevent major changes in pH. Small or localized changes in pH are quickly modified by various chemical reactions, so little or no change may be measured. This ability to resist change in pH is called buffering capacity. Not only does the buffering capacity control would-be localized changes in pH, it controls the overall range of pH change under natural conditions. Expected Impact of Pollution When pollution results in higher productivity (increased growth in aquatic plants or algae), pH levels may increase. Although these small changes in pH are not likely to have a direct impact on aquatic life, they greatly influence the availability and solubility of all chemical forms in the lake and may aggravate nutrient problems. For example, a change in pH may increase the solubility of phosphorus, making it more available for plant or algae growth and result in a greater longterm demand for dissolved oxygen or excessive plant and algae growth. Lakes or streams that have received too much rain with a low pH (acid rain) lose their buffering capacity. Once the buffering capacity of a stream or lake is lost, change occurs relatively quickly. Each river or lake has its own unique range of pH which varies daily and seasonally but the pH of our natural waters usually hovers between 6.5 and 8.5. Although in the spring it is not uncommon to see pH values as low as 5.5 in streams that run through heavily forested areas. This is caused by the release of tannic acid from rotting wood. Some types of plants and animals are able to tolerate acidic waters. Others, however, are acidsensitive and will be lost as the pH declines. Generally, the young of most species are more sensitive to environmental conditions than adults. At pH 5, most fish eggs cannot hatch. At lower pH levels, some adult fish die. Some acid lakes have no fish. The chart below shows that not all fish, shellfish, or the insects that they eat can tolerate the same amount of acid; for example, frogs can tolerate water that is more acidic (i.e., has a lower pH) than trout. Conductivity Conductivity is the ability to conduct electricity. Water conducts electricity because it contains dissolved solids that carry electrical charges. For example, chloride, nitrate, and sulfate carry negative charges, while sodium, magnesium, and calcium carry positive charges. These dissolved solids affect the water’s ability to conduct electricity. Most streams have a fairly constant range of conductivity under normal circumstances. Therefore, significant changes in conductivity can be an indicator that a discharge or some other source of pollution has entered the water. The composition of the water can be critical for aquatic organisms as well, as many critters have very specific ranges that they can tolerate. Conductivity can be affected by many factors. Examples include: •The addition of fresh water (rain) lowers conductivity because rainwater has low conductivity and the increase in water levels dilutes mineral concentrations. Conversely, during low flow conditions (summer and fall) the dissolved solids are more concentrated and therefore conductivity levels are higher. •Conductivity is affected by temperature: the warmer the water, the higher the conductivity. •Soil and rocks release dissolved solids into the waters that flow through or over them. Therefore, the geology of a certain area will determine the conductivity. •In coastal streams or estuaries, salt water often mixes with fresh water. The addition of salt water greatly increases conductivity. Typical conductivity ranges in our water resources are: Distilled Water: 0.5 to 3.0 mS Melted Snow: 2 to 42 mS Drinking Water: 30 to 1500 mS Freshwater Streams: 100 to 2000 mS TDS or Total Dissolved Solids. This refers to the amount of dissolved ions, minerals, salts, metals and other chemicals in water. The amount is expressed in mg/L or parts per million (ppm). Pure water has virtually no conductivity of electricity. But the more TDS rises in water the greater the conductivity of the water becomes to electricity and so the value of TDS can be measured and expressed by a electronic device (called a TDS Meter) which passes a minute current through it and gives you a readout which is in mg/L or ppm. This reading is directly related to the purity of the water. So the higher the reading the more solids are dissolved in the water. TDS in mg/L 0 50 100 200 300 400 500+ 0-50 from distilled water (very soft) (minerals have been removed) 50-150 water from carbon filtration (soft) . 150-300 typical human taste acceptable tap water (medium to hard). 300-500 Unpleasant tasting hard water (hard to very hard). 500+ Unacceptable for human consumption. High concentrations of TDS will reduce water clarity, contribute to a decrease in photosynthesis and lead to an increase in water temperature. It will make water cloudy and cloudy water will absorb heat from the sun faster than clear water, raising water temperatures. Cloudy water also inhibits aquatic organisms in their search for food and nesting habitat. Water becomes electrically charged once it reaches a TDS count of 50 ppm, meaning it can conduct electricity at this point. In addition, regulations on drinking water standards dictate that an upper limit of 500 mg/l of TDS must be held for drinking water. Higher levels can be a contributing factor for corrosion in plumbing and affects clothes washing. The aesthetic quality of water is also disrupted at levels higher than 500. Nitrates Nitrogen occurs in natural waters as nitrate (NO3), nitrite (NO2), ammonia (NH3), and organically bound nitrogen. Of these, nitrate is usually the most important to consider when determining water quality. Normally only small amounts are found naturally, such as those formed when aquatic plants and animals die: bacteria break down large protein molecules containing nitrogen into ammonia. Ammonia is then oxidized by specialized bacteria to form nitrites and nitrates. An increase in nitrate levels can come from numerous man-made sources such as septic systems, fertilizer runoff and improperly treated wastewater. It also occurs naturally when plants or animals die and their nutrients are released through decomposition. Nitrates are one of the nutrients that plants and algae need to grow. If nitrates are too plentiful in an aquatic ecosystem plants and algae can take over. When excessive amounts of plants and algae die, the bacteria that decomposes them can use up all of the available oxygen in water. This will deprive all other aquatic organisms of oxygen and sometimes results in massive fish kills. An increase in nitrates may accompany an increase in phosphates. As phosphates increase and the growth of aquatic plants is encouraged, algal blooms can occur. With the increase in algae growth and decomposition, dissolved oxygen levels will likely decrease. Sources of phosphates include septic tanks, runoff from feedlots, runoff from agricultural operations and waste water and sewage treatment plants. The water quality guidelines in Canada state that in order to protect freshwater life, levels of nitrates should be no higher than 13 mg/L (Environment Canada, 2005). Phosphorous Pure phosphorus (P) is rare. In nature, phosphorus usually exists as part of a phosphate molecule (PO4). Phosphorus in aquatic systems exists as organic phosphate and inorganic phosphate. Organic phosphate consists of a phosphate molecule associated with a carbon-based molecule, as in plant or animal tissue. Phosphate that is not associated with organic material is inorganic. Inorganic phosphorus is the form required by plants and is known as orthophosphate. Plants require orthophosphate for photosynthesis, making orthophosphate a limiting factor in aquatic plant growth. Since phosphorus is a nutrient normally in short supply in most fresh waters, even a modest increase in phosphorus can, under the right conditions, set off a whole chain of undesirable events in a stream including accelerated plant growth, algae blooms, low dissolved oxygen, and the death of certain fish, invertebrates, and other aquatic animals. Orthophosphate forms of phosphorous are produced naturally, but can also be introduced in streams by man-influenced sources such as: partially treated and untreated sewage, runoff from agricultural sites, and application of lawn fertilizers. Orthophosphates applied to agricultural or residential lands as fertilizers are carried into the surface water during storm events or snow melts. Currently, no national environmental quality guidelines exist in Canada for phosphorus, although individual provinces may have guidelines or objectives (Environment Canada 2004). The Federal criteria for phosphorous levels in the USA are: 0.1 mg/L for streams and rivers, 0.05 mg/l for streams entering lakes 0 .025 mg/l for lakes and reservoirs. http://www.water.ncsu.edu/watershedss/info/phos.html Water Temperature Water temperatures have a direct influence upon the composition of aquatic communities (Bain and Stevenson,1999). Fish can only survive and thrive within a limited temperature range, Brook Trout for example require cooler temperatures, ideally within the range of 13–18 °C; however they are able to survive in water temperatures of up to 22 °C. The temperature of the water can vary with time of day, the season, and the water depth. Heat gains and losses occur more rapidly in small streams where the water depth is shallow and is intermittently mobile. Although water temperatures are dependent on direct solar radiation, they can also be influenced by: Water velocity Climate Elevation Amount of stream side vegetation providing shade Temperature and volume of groundwater input (presence of springs) The dimensions of the stream channel Human impact Sediment and dissolved solids - Runoff can indirectly add warm water to streams. Rainwater running off from heated surfaces such as parking lots, roof tops and roads has been documented to increase stream temperatures (Rossi, L. and RE. Hari. 2007). Dissolved Oxygen An important measure of water quality is the amount of dissolved oxygen (DO) that can be found in an aquatic ecosystem. Higher levels of DO generally indicate a healthy and stable ecosystem capable of sustaining many different kinds of aquatic life. Different organisms require different levels of DO; salmonids require high levels of DO (7-14 mg/L), while carp and catfish can survive in waters with low levels of DO (below 7 mg/L). The DO levels can also influence aquatic insects. Recommended dissolved oxygen levels in (mg/L), (Adapted from S. Behar. 1996) 0-2 mg/L: Not enough oxygen to support life 2-4 mg/L: Only a few kinds of fish and insects can survive 4-7 mg/L: Acceptable for warm water fish (carp, goldfish, catfish, bass) 7-11 mg/L: Very good for most stream fish including cold water fish (trout, salmon) The Canadian water quality guideline for the protection of aquatic organisms is a minimum of 5.5 mg/L for DO in freshwater ecosystems. There are several factors that can influence the amount of DO present in a body of water: (1) Temperature: Water Temperature and DO are directly related to each other. As water temperature increases, DO decreases, thus cold water holds more oxygen than warm water. (2) The level of photosynthesis (by plants and algae submerged in the water.) (3) Flow rate and turbulence: Flow and DO are directly related to each other. Higher volumes of faster moving water, especially if it creates "white water" increases the turbulent diffusion of atmospheric oxygen into the water. Low flow conditions are much less conducive to oxygenation and when water temperature is high, DO levels can become critically low. (4) Degree of light penetration as determined by turbidity and water depth. (5) Amount of decaying organic matter and nutrients: As the amount of organic material and excessive nutrients increases, the biochemical oxygen demand (BOD) tends to increase. Dissolved oxygen is used up in the decomposition of organic matter, which in turn lowers the available oxygen for organisms in an aquatic ecosystem.