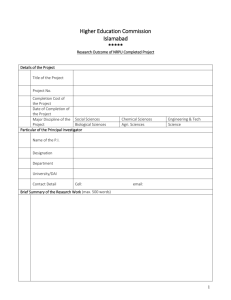
Session Open to abstract submission
advertisement

INDUSTRY SYMPOSIUM Session: open to abstract submissions Translational Research of ATMPs clinical / commercial translation Chairs: Rui Amandi da Sousa, Vincent Ronfard, Yves Bayon Regenerative medicine breakthrough ideas and innovations, given to birth by academic scientists, when carefully evaluated, may have obvious and huge benefit for patients and the public, by satisfying massive unmet clinical needs, for a still large range of indications where no therapeutic approaches are really effective, today, in restoring an enjoyable level of quality of life: neurological (eg. stroke, spinal cord injury), orthopedic (eg. intervertebral disc defects, large cartilage lesions), cardiovascular (eg. critical limb ischemia, congenital heart disease, myocardial infarction), endocrine (eg. diabetes), renal (end stage kidney disease), gastrointestinal (eg. Crohn’s disease, ulcerative colitis), skin (eg. diabetic foot ulcer, venous keg ulcers) indications… But, the decision to move forward regenerative medicine discoveries into the clinic and to the market is certainly not an easy one. The path leading to commercialization – from promise to real delivery, from prototype to useful product. – is indeed challenging, with a number of obvious hurdles to overcome: eg. creating a business activity, financing, patent filing, regulatory requirements, flexible manufacturing development, clinical demonstration, partnership / alliance management… However, meeting and learning from key academia and early stage companies, sharing their ATMP translation may certainly benefit all attendees, having running, intending to run or interested in learning more on translation projects. Actively participating to this session may also give a chance to interact with the audience, a mix of academia and companies and to establish solid contacts for future relationships. With most funding sources pressuring and the complexity of the commercialization process of advanced therapies, there is an obvious need to increase the speed to the market, but without increasing risk. This may be achieved by pooling know-hows and experiences. This session, a part of Industry symposium, aims to highlight the translational research efforts or projects of in-house developed advanced therapies, even at an early stage, managed by academic and/or academic spin-off companies and to discuss key technical/scientific, legal/IP, financing, regulatory and clinical advancements and current hurdles paving the road of the early stage commercialization process.