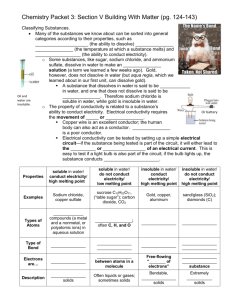
Light Up My Life Lab
advertisement
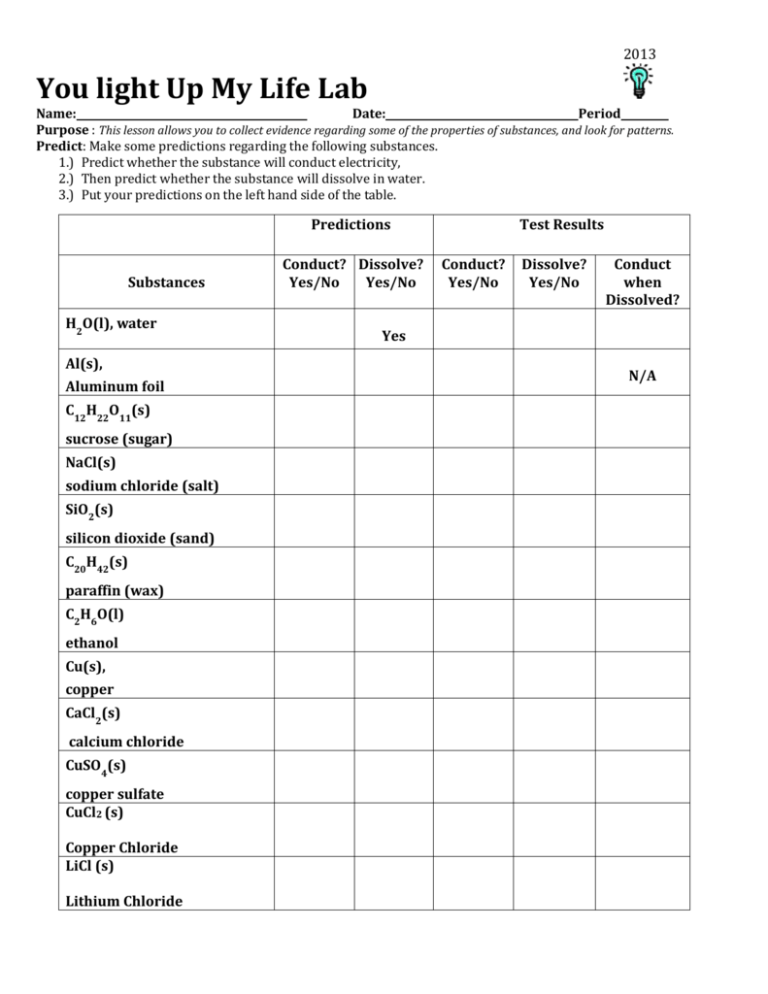
2013 You light Up My Life Lab Name: Date: Period Purpose : This lesson allows you to collect evidence regarding some of the properties of substances, and look for patterns. Predict: Make some predictions regarding the following substances. 1.) Predict whether the substance will conduct electricity, 2.) Then predict whether the substance will dissolve in water. 3.) Put your predictions on the left hand side of the table. Predictions Substances H2O(l), water Al(s), Aluminum foil C12H22O11(s) sucrose (sugar) NaCl(s) sodium chloride (salt) SiO2(s) silicon dioxide (sand) C20H42(s) paraffin (wax) C2H6O(l) ethanol Cu(s), copper CaCl2(s) calcium chloride CuSO4(s) copper sulfate CuCl2 (s) Copper Chloride LiCl (s) Lithium Chloride Conduct? Dissolve? Yes/No Yes/No Test Results Conduct? Yes/No Dissolve? Yes/No Conduct when Dissolved? Yes N/A 2013 Procedure: 1. Apparatus. Assemble your conductivity apparatus correctly. Connect battery to wire snap. You will use this apparatus to test the conductivity of the various substances in the table. Then enter your results in the right hand side of your table. 2. First, test the solid substance in the tray to determine if it will conduct electricity. Record your answer in the table. 3. Then you need to determine if the substance dissolved in water. IF YOUR SUBSTANCE DISSOLVES IN WATER, TEST THE LIQUID WITH YOUR CONDUCTIVITY APPARATUS. IF IT DOES NOT DISSOLVE, THEN YOU WILL NOT TEST THE LIQUID. Record your results in your table. 4. When the substance does not dissolve in water, enter N/A in the last column for Not Applicable. Wipe off your paper clips after each use. (Oxidation will occur) Use your data Post Lab Questions: 1. Make a list of the substances that conduct electricity, but do not dissolve in water. What do those things have in common? (Hint* think about their properties) 2. Divide the substance that dissolve in water into two categories. Those that conduct electricity once dissolved, those that don’t conduct electricity. Substances that dissolve in water Conduct Don’t Conduct 3. What are three things the substances that conduct electricity, once they are dissolved in water, all have in common? 4. What do the substances that do not conduct electricity once they are dissolved all have in common? (leave water out) Summarize: Explain your test results and answer the questions below. 5. What can you now conclude about substances the do not light up the light bulb in this lab? Complete the following sentence. The substances that do not light up the light bulb… 6. What can you now conclude about substances the do light up the light bulb in this lab? Complete the following sentence. The substances that do light up the light bulb… 7. Predict whether isopropanol, C3H8 O(l), will conduct electricity. Explain your reasoning.