CASE FOR C2-Respiratory System Eric Suarez and Barbara
advertisement
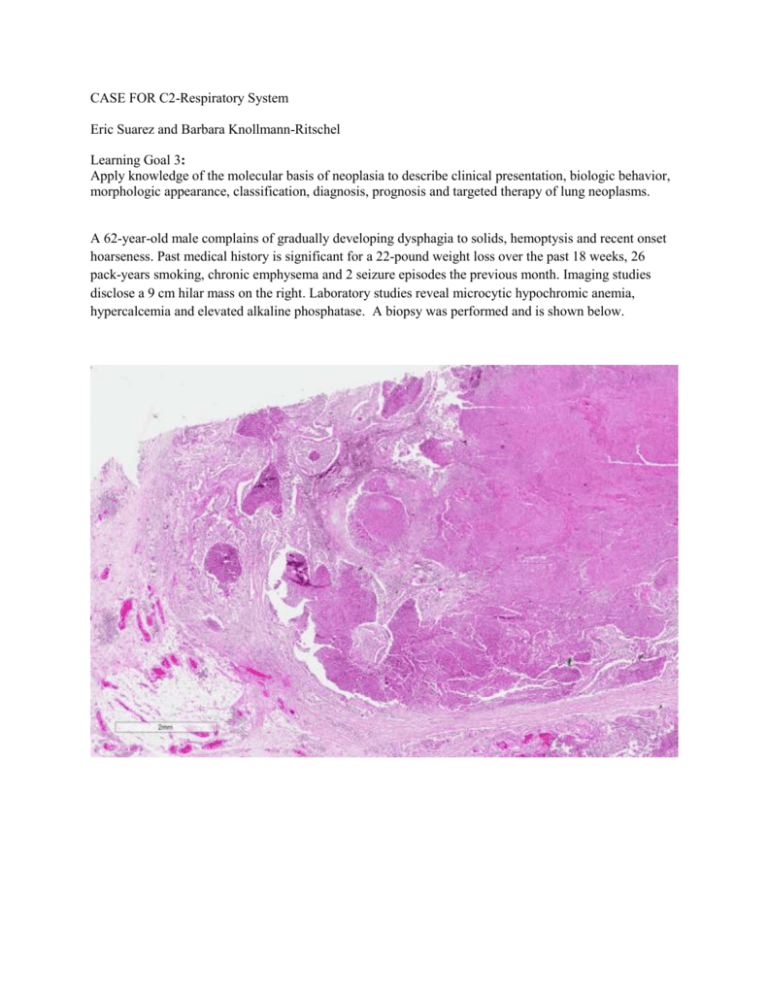
CASE FOR C2-Respiratory System Eric Suarez and Barbara Knollmann-Ritschel Learning Goal 3: Apply knowledge of the molecular basis of neoplasia to describe clinical presentation, biologic behavior, morphologic appearance, classification, diagnosis, prognosis and targeted therapy of lung neoplasms. A 62-year-old male complains of gradually developing dysphagia to solids, hemoptysis and recent onset hoarseness. Past medical history is significant for a 22-pound weight loss over the past 18 weeks, 26 pack-years smoking, chronic emphysema and 2 seizure episodes the previous month. Imaging studies disclose a 9 cm hilar mass on the right. Laboratory studies reveal microcytic hypochromic anemia, hypercalcemia and elevated alkaline phosphatase. A biopsy was performed and is shown below. 1. Based on the histologic findings and clinical presentation, what is your diagnosis? Squamous cell carcinoma On low power there are multiple infiltrating nests of tumor cells as well as a large area of necrosis in the upper right corner of the first image above. On higher power, there are sheets of polygonal cells present with a high nuclear to cytoplasmic ratio, hyperchromatic and pleomorphic nuclei and large keratinizing cells in the necrotic area. The keratinization can be in the form of keratin pearls or deeply eosinophilic cytoplasm of the malignant cells as seen in the necrosis in the third image above. 2. If the tumor above spreads locally to the recurrent laryngeal nerve, which clinical feature would you elicit in the patient history? Hoarseness Local spread of lung tumors produce multiple clinical features. Over 50% of patients will present with a cough if the tumor involves the central airways, 24-50% will present with hemoptysis if there is hemorrhage secondary to tumor invasion in the airway, hoarseness if there is recurrent laryngeal nerve invasion, diaphragm paralysis with phrenic nerve invasion, and pleural effusions with pleural invasion. (Reference: Robbins and Cotran Pathologic Basis of Disease, 9th edition, p 718). 3. Chronic smoking is a well-known carcinogen associated with lung cancer. Still, lung cancer only develops in 11% of heavy smokers. What genetic risk factors most commonly promote developing squamous cell carcinoma within this subgroup? TP53 mutations and cytochrome p450 polymorphisms Carcinogenesis is the process by which normal cells are transformed in carcinoma. This is often a long process in which multiple mutations are induced in a sequence. These mutations can include initiator mutations leading to genomic instability, which is followed by additional mutations over a longer period of time leading to the “founding cancer cell”. As the tumor continues to grow, cells compete for nutrients in the microenvironment and there is a tendency toward additional mutations in tumor progression leading to genetically heterogeneous tumors. (Reference: Robbins and Cotran Pathologic Basis of Disease, 9th edition, p 280-281). In lung tumors, the duration of smoking, and the amount of exposure to cigarette smoke is well associated with lung cancer risk. In cigarettes particularly, there are multiple procarcinogens present which can be converted to carcinogens via the P-450 monooxygyen system. In some patients there are polymorphisms present in the P-450 system which have an even greater propensity to activate these procarcinogens to carcinogens in cigarette smoke in patients that harbor these genetic variations of the P-450 system. In addition, the normal mucosa cells of patients with lung squamous cell carcinoma can have mutations of TP53. This mutation is seen as an early event in the progression to squamous cell carcinoma and most commonly in squamous cell carcinoma when compared to other histologic variants of lung cancer. It is found in nearly half of the cells of squamous cell dysplasia, and almost all cells in carcinoma in situ. (Reference: Robbins and Cotran Pathologic Basis of Disease, 9th edition, p 712-5). 4. Which of the findings described in the patient presentation above is most closely associated with the specific histologic type of the patient’s tumor? Hypercalcemia This is an example of a paraneoplastic syndrome. Paraneoplastic syndromes are presentations in the patient’s clinical history that do not readily relate to the anatomic location or spread of a tumor, rather are common findings secondary to hormonal production or immunologic events. Paraneoplastic syndromes important to recognize for several reasons including: these may cause significant clinical consequences, such as hypercalcemia, this may be the first clinical sign of on underlying malignancy, or they may confuse the clinical picture. Hypercalcemia is a common paraneoplastic syndrome that can be seen with multiple different types of neoplasms including squamous cell carcinoma of the lung, breast carcinomas, renal cell carcinoma, ovarian neoplasm or adult t-cell leukemia/lymphoma. The mechanism of the production of the hypercalcemia is due to PTHRP production which can be induced by cancer in the bone metastasis, or it may be related to other factors secondary to the malignancy such as TGF-a, TNF, or IL-1. Other common paraneoplastic syndromes include polycythemia secondary to erythropoietin production as seen in renal cell carcinoma, or hypoglycemia due to insulin or insulin-like substance production as seen in ovarian carcinomas, or Cushing syndrome due to ACTH production as seen in small cell carcinoma of the lung. (Reference: Robbins and Cotran Pathologic Basis of Disease, 9th edition, p 330-331).