File
advertisement

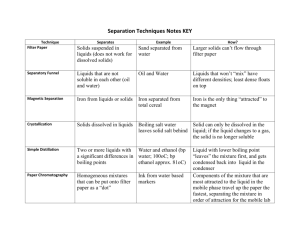
Misconception 1 All materials can be classified as a solid, liquid or gas. (Department of Education and Training, 2015). About Research suggests conceptual understandings of solids, liquids and gases are shaped early on through everyday interactions and language appears to strongly influence their identification of each state (for eg. students identify solids as being able to be held, liquids as runny and gases like air (Australian Science Teachers Association, 2013). These experiences create the misconception that matter can only exist or have properties of one form and are easily categorised (Jones, 1984 & Krnel, Watson & Glazar, 1998). McGuigan, Qualter & Schilling (1993) says when identifying a liquid, students generally select options such as water, milk, and other water based solutions. McGuigan et al (1993) says rarely students identify non-water based liquids such as oil, paints, or detergent and generally relate liquid to water and or that all liquids contain water. This misconception is complicated by the belief solids must be hard, substances such as powder or a sand are a liquid as they can be poured or that powder is solid because it does not ‘wet’ (McGuigan, Qualter & Schilling, 1993, Krnel, Watson & Glazar, 1998). Scientific view Human nature encourages categorising. It is easy to see how this misconception occurs as a result of students’ life experience. Kyle, Family, & Shymansky (1989) says these notions need to be challenged by giving encountering experiences which challenge these perceptions. The three levels of classification (solids, liquids and gas) are mandated by the year 5 level curriculum and provide a convenient framework. However, many materials make classification troubling such as powder, toothpaste, the human body, gel, goo, dry ice, dripping icy poles, a soft drink can, candle wax, mayonnaise and so on. The Department of Education and Training (2014) provides principles to assist with the classification which can be found here. Classification is further hindered by substances which change states depending on factors such as temperature, pressure or multiple states such as chocolate, butter and immersions (Department of Education and Training, 2014). Links to the Australian Curriculum (ACARA, 2015) Year Level Year 5 Content Description Science Understanding (Chemical Science) Solids, liquids and gases have different observable properties and behave in different ways (ACSSU077) recognising that substances exist in different states depending on the temperature observing that gases have mass and take up space, demonstrated by using balloons or bubbles Elaborations exploring the way solids, liquids and gases change under different situations such as heating and cooling recognising that not all substances can be easily classified on the basis of their observable properties Teaching Strategies and Suggestions Critical teaching ideas 0. Classifying similar characteristics 1. Solids, liquids and gases are the overarching categories however some materials and substances do not clearly fit within these parameters 2. Sometimes multiple classifications can be applied 3. Classification can be affected by multiple factors NOTE: Students have extensive prior knowledge from the Chemical Science strand of the Australian Curriculum (2015) on states of matter, the effects of temperature and physical and chemical change. Engage 4. Class jigsaw: split into three groups with a pen and butcher paper labelled ‘what is a solid’, ‘what is a liquid’ and ‘what is a gas’. Each group must brainstorm and then regroup to present findings and record results. This will inform the teaching process for what they do and don’t know (Australian Science Teachers Association, 2013). Explore 5. Use POE (predict, observe and explain) chart. Produce a mystery bag containing solids, liquids and gasses. Fill out prediction column. Hand items around then complete observation. Use elbow partners to build understandings and discuss observable features. Include easily identifiable items as well as tricky ones. Discuss results as a class and complete the explain column. Click below to print posters for sorting. The misconception is challenged by sorting items into the categories including items which don’t obviously belong anywhere (Australian Science Teachers Association, 2013). (Idea sourced from http://scienceweb.asta.edu.au/years-5-6/unit1/lesson-one/yr56-unit1-lesson-1.html) 6. Conduct an investigation on the life cycle of a snowman to investigate the physical change of water (ice, water then to gas). Using a snowman provides a real life example. Classify the snowman by creating a hypothesis, making predictions, taking scientific observations throughout the day including the state of matter, create a conclusion explaining what happened and why. Activity information and instructions: http://www.frugalteacher.com/2011/09/life-cycle-of-snowman.html. Reinforce investigation by watching the video on solids, liquids and gases: https://youtu.be/jd0RXHfIKJQ. Discuss the effects of temperature on matter and how it affects the state. Discuss whether water is a solid, liquid or gas. Draw a diagram. This task demonstrates how temperature makes classification difficult thus negating the misconception (The Association for Science Education, 2008). 7. Create slime: http://scienceweb.asta.edu.au/years-5-6/unit1/lesson-five/yr56-unit1-lesson-five.html and complete the investigation attached explaining the behaviour of pressure or force and its state. What kind of change has it undergone? This task demonstrates how pressure makes classification difficult thus negating the misconception (The Association for Science Education, 2008). Explain 8. Work though ‘What the world is made of’ (whole class, small groups or individually) covering states of matter and changes to the state of matter: http://www.scootle.edu.au/ec/viewing/L3249/index.html (Scootle, 2015). This challenges the misconception and provides extra information in a real life context. Elaborate 9. Download the poster, display and use to conduct a simulation role play where students move like a molecule. Provide examples which cannot simply be classified as a solid, liquid or gas. Students pick one and draw a diagram as well as explain everything they can about the classification process and why it is difficult. https://s-media-cacheak0.pinimg.com/736x/b7/f0/aa/b7f0aa9a0d0cb79cc7fa066cc12da301.jpg Evaluate Students choose a format (written, typed, recorded etc) to define a solid, liquid and a gas. Provide 3 examples of materials or substances which are hard to classify and why. Finally what factors can influence classification (temperature, force, physical change or chemical change). Students select one object/material/substance and create a presentation on how to classify it, the difficulty with classifying and determining the factors.