answer to first h.w
Nicotinamide adenine dinucleotid
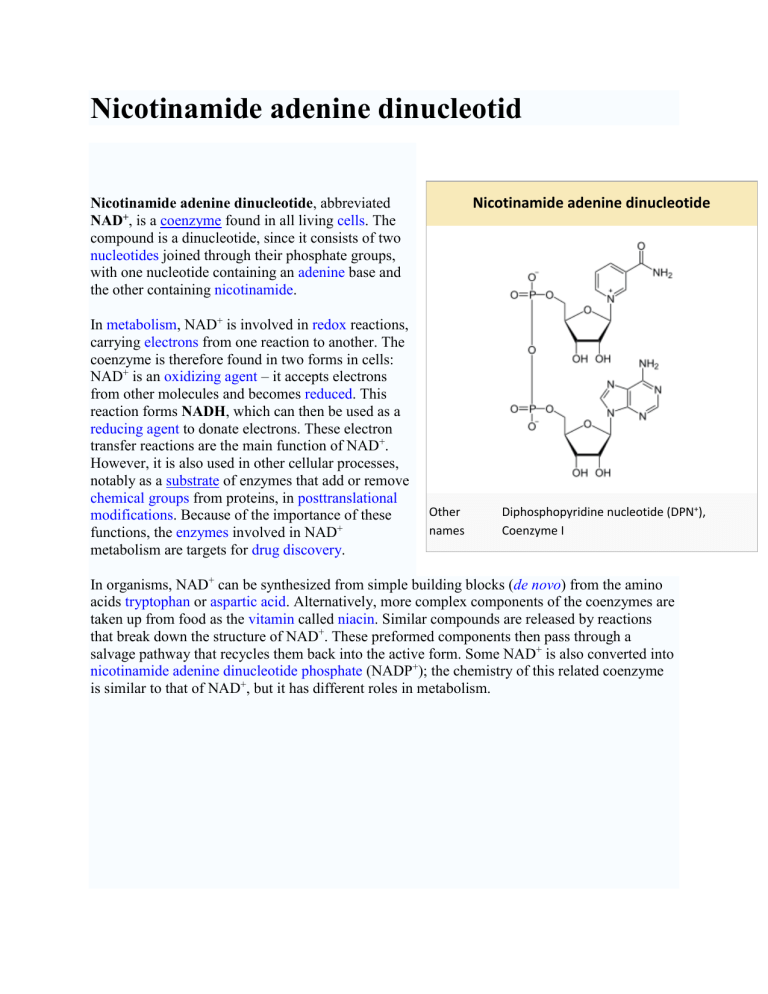
Nicotinamide adenine dinucleotide , abbreviated
NAD + , is a coenzyme found in all living cells . The compound is a dinucleotide, since it consists of two nucleotides joined through their phosphate groups, with one nucleotide containing an adenine base and the other containing nicotinamide .
In metabolism , NAD
+
is involved in redox reactions, carrying electrons from one reaction to another. The coenzyme is therefore found in two forms in cells:
NAD
+
is an oxidizing agent – it accepts electrons from other molecules and becomes reduced . This reaction forms NADH , which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD
+
.
However, it is also used in other cellular processes, notably as a substrate of enzymes that add or remove chemical groups from proteins, in posttranslational modifications . Because of the importance of these functions, the enzymes involved in NAD + metabolism are targets for drug discovery .
Other names
Nicotinamide adenine dinucleotide
Diphosphopyridine nucleotide (DPN + ),
Coenzyme I
In organisms, NAD
+
can be synthesized from simple building blocks ( de novo ) from the amino acids tryptophan or aspartic acid . Alternatively, more complex components of the coenzymes are taken up from food as the vitamin called niacin . Similar compounds are released by reactions that break down the structure of NAD
+
. These preformed components then pass through a salvage pathway that recycles them back into the active form. Some NAD + is also converted into nicotinamide adenine dinucleotide phosphate (NADP
+
); the chemistry of this related coenzyme is similar to that of NAD
+
, but it has different roles in metabolism.
FAD
In biochemistry , flavin adenine dinucleotide ( FAD ) is a redox cofactor involved in several important reactions in metabolism . FAD can exist in two different redox states and its biochemical role usually involves changing between these two states. Many oxidoreductases , called flavoenzymes or flavoproteins , require FAD as a prosthetic group which functions in electron transfers . FADH is known as reduced flavin adenine dinucleotide.
FAD
FAD is derived from riboflavin (vitamin B
2
). It consists of a riboflavin group bound to the phosphate group of an adenosine diphosphate molecule. Note that, though the name flavin adenine dinucleotide is a misnomer (the molecule contains only one nucleotide , the riboflavin moiety is not linked to the D-ribityl group through glycosidic bond ),
[1]
it is generally accepted now.
FAD can be reduced to the FADH
2
, whereby it accepts two hydrogen atoms:
The reduced coenzyme FADH
2
is an energy-carrying molecule, and it can be used as a substrate for oxidative phosphorylation in the mitochondria . FADH
2
is reoxidized to FAD, which produces enough of a proton gradient across the inner mitochondrial membrane for the enzyme ATP synthase to produce 2.0 equivalents of the high-energy compound ATP . The primary sources of reduced FAD in eukaryotic metabolism are the citric acid cycle and the beta oxidation reaction pathways. In the citric acid cycle, FAD is a prosthetic group in the enzyme succinate dehydrogenase that oxidizes succinate to fumarate , whereas in beta oxidation it serves as a coenzyme in the reaction of acyl CoA dehydrogenase .
Glutathione
Glutathione
[1]
Glutathione ( GSH ) is a tripeptide . It contains an unusual peptide linkage between the amine group of cysteine and the carboxyl group of the glutamate side chain . Glutathione, an antioxidant , helps protect cells from reactive oxygen species such as free radicals and peroxides .
[2]
Glutathione is nucleophilic at sulfur and attacks poisonous electrophilic conjugate acceptors .
Thiol groups are kept in a reduced state at a concentration of approximately ~5 mM in animal cells . In effect, glutathione reduces any disulfide bond formed within cytoplasmic proteins to cysteines by acting as an electron donor. In the process, glutathione is converted to its oxidized form glutathione disulfide (GSSG). Glutathione is found almost exclusively in its reduced form, since the enzyme that reverts it from its oxidized form, glutathione reductase , is constitutively active and inducible upon oxidative stress . In fact, the ratio of reduced glutathione to oxidized glutathione within cells is often used scientifically as a measure of cellular toxicity