Cell Biology – Scientific Literacy
advertisement

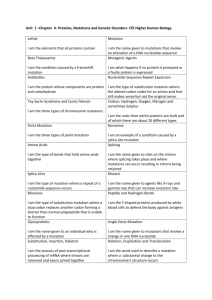
Brittany Niece Writing Assignment Alpha-synuclein (αS) is an abundant protein found in the neurons of the brain that is thought to play a key role in synaptic vesicle transmission. In our brains, we have presynaptic terminals at the end of every neuron; it is here that αS interacts with lipids and proteins to, as it is suggested, maintain levels of synaptic vesicles these terminals. It is worth noting that αS is thought to regulate the dopamine levels of the brain. Dopamine is a critical neurotransmitter responsible for voluntary and involuntary movements of the musculoskeletal system, which, when misfolded or present in toxic amounts, can impair neuron function or even kill the neuron completely. The importance of this is to help understand the uncontrolled and twitch-like movements of someone diagnosed with Parkinson's disease or Multiple System Atrophy. With aging and certain neurodegenerative diseases, such as those mentioned and dementia with Lewy bodies, the αS protein can improperly fold and cluster into aggregates which are insoluble in neurons due to "missense mutations, copy number variants, or up-regulated expression" (Dettmer). Although it was initially believed to strictly form as an unfolded monomer, it has been discovered that αS can also form "physiological multimers, principally tetramers, which have α-helical conformation" (Dettmer). This infers that αS can form multimers which are soluble and do not cause any mutation or neurodegenerative diseases; the physiological forms are distinct from the pathological forms (Beta-sheet rich aggregates). Beta-synuclein (βS), the homolog of alpha-synuclein, exhibit cell lysis sensitivity in chemical cross-linking experiments, which suggests "that a dynamic intracellular population of metastable αS multimers and monomers coexist normally" (Dettmer). The importance of alpha-synuclein is proven through this minimal information: if there is too much present, or if αS is present as aggregated clumps throughout the brain, there will be serious neurodegenerative complications, whether they be mental, musculoskeletal, or both. The structure of KTKEGV, as noted in the image below, is polar, charged, and mostly hydrophilic, having only two nonpolar amino acids in the polypeptide chain. These finding indicate that αS will be found on the surface of the protein. Of note: KTKEGV has a net +1 charge. The secondary structure of normal αS has long been thought to be an unfolded monomer; however, through recent research, it has come to light that these proteins can also take on alpha-helical conformations forming multimers and tetramers, which are referred to simply as multimers. Pathological alpha-synuclein, on the other hand, was found to have a secondary structure described as beta-sheet rich aggregates which are called oligomers. From attempts to research further, it is noted that while tertiary and quaternary forms “…could provide some insight into the pathogenic mechanism associated to point mutations,” there is still no solid evidence that they exist (Kara et al.). In their article, Kara et al. state that there have been studies suggesting that a tertiary structure of “…α-synuclein consists of two antiparallel α-helices linked through a short protein chain that naturally assembles into tetramers presumably preventing α-synuclein monomer aggregation,” but again, there is not much evidence supporting this. Dettmer et al. want to show that a mutation causing the abrogation, or retraction, of αS tetramers can cause the very insolubility, inclusions, and neurotoxicity of αS that causes neurodegenerative diseases. Therefore suggesting that the “folded tetrameric form is indispensable for normal αS homeostasis” (Dettmer). That is to say that this folded tetrameric form of alpha-synuclein actually lessens the likelihood of mutations leading to the negative neurodegenerative effects. Based on the common recurrence of each amino acid at each position, it is clear why the particular sequence, KTKEGV, is used as the reference sequence. In more than half of the 9 samples, each amino acid is found in the same position. This leads to the idea that it is always more likely for that particular amino acid to appear in that location pending possible mutations. For each mutation, the charge, size, or polarity of the amino acid changed. For ease of understanding, the different mutations used in this experiment are listed below: a. KTKKGV – this mutation of E to K caused a change in the charge of the amino acid (negative to positive), thus affecting the net charge of the whole polypeptide chain (+1 to +3). This mutation deferred the formation of tetramers. b. KTEEGV – this mutation of K to E also caused a change in the charge of the amino acid, but it went from positive to negative this time, giving the whole chain a net charge of -1. This mutation still allowed for the formation of tetramers. c. KTKEIV – inserting I in place of G caused the polypeptide chain to become larger. This mutation deferred the formation of tetramers. d. GTKEGV – the charge of the amino acid G is 0, whereas the charge of K is +1. This mutation causes the net charge to be 0. This mutation still allowed for the formation of tetramers. e. KLKEGV – switching the T for L changes the polarity, with T being a polar uncharged amino acid, and L being hydrophobic. This mutation deferred the formation of tetramers. f. KTKEGR – this switch also affects the polypeptides polarity along with its size and charge. V is a small hydrophobic amino acid while R is much larger, hydrophilic, and positively charged (net charge = +2). This mutation still allowed for the formation of tetramers. g. KTKEGW – mutating the V to a W changes the size, causes the mutant to be larger. This mutation deferred the formation of tetramers. Only several of the observed mutations were cytotoxic; KTKKGV, KLKEGV, KTKEIV, and KTKEGW. It appears that increasing the size, inducing a positive charge, and altering the polarity of the sequence plays a key role in ceasing the formation of tetramers. First looking at living cells, it is noted that there are no inclusions of the αS protein present. Comparing this finding to several of the mutants, (KLK, KGV, EIV, EGR, and EGW) shows significant differences in all mutants except EGR, which also has no significant finding for αS inclusions. For KLK, KGV, EIV, and EGW however, it shows a similar percentage (roughly 100%) of inclusions in the living cells, with KGV only slightly lesser than the others. Of note: these four mutants are also the only four tetramer abolishing mutants in the study. The importance of these inclusions is that they are signs of neurodegenerative diseases. Based on review of the experiments, there appears to be a pattern displayed within the various mutations. When amino acid size was increased, it caused the αS protein to become larger, thus eliminated tetramer formation - causing the protein to be cytotoxic. The same is seen by increasing the net charge, altering the position of hydrophobic versus hydrophilic amino acids within the polypeptide chain. When the size, net charge, and polarity of αS was decreased, it still allowed the mutants to form tetramers. The only exception to the pattern is in the EGR mutant: as previously stated, V is a smaller, hydrophobic amino acid that is uncharged, while R is larger, hydrophilic, and has a charge of +1. This finding would lead to the assumption that this mutant would abolish tetramer formation, however, it displays no such behavior. The authors’ research does support their hypothesis. It is clearly proven that the abrogation of tetramers leads to cytotoxicity in cells, inevitably leading to neurodegenerative diseases. Through this research, scientists can now work on methods to identify and alleviate αS tetramers and multimers, and “place multimerization of this abundant neuronal protein into a functional context” (Dettmer). In other words, it has now been discovered how this protein mutates, what can come from these mutations, and how these mutations appear in cells; therefore, further research and understanding of these proteins could likely lead to treatment or a cure. The discovery of what regulates the αS monomeric and tetrameric/related multimeric homeostasis is the missing piece keeping a treatment or cure out of reach. Knowing that this protein is a prion leads to the concern for transmission through deep-brain stimulation equipment used to treat Parkinson’s disease (PD). The concern stems from the fact that other neurodegenerative diseases, such as multiple system atrophy (MSA), can be initially diagnosed as PD. In an article, the University of California quotes a published researcher stating, “"You can't kill a protein…And it can stick tightly to stainless steel, even when the surgical instrument is cleaned."” Therefore, if a patient with undiagnosed MSA is treated for PD with the same equipment used for other patients, the prions could be transferred from patient to patient. Another step into future research concerning αS proteins is in the possibility of pharmacological treatments. In Reynaud’s article, Protein Misfolding and Degenerative Diseases, he mentions that “therapeutic inhibition of precursor protein synthesis is within reach…drugs that induce chaperone expression are also being tested, as well as inhibitors that prevent protein hyperphosphorylation.” Reynaud also notes that “…scientists have more options to find common structures for the design of specific chemical inhibitors of aggregation…” with research providing knowledge of progressively more amyloid beta sheet structures. Lastly, Reynaud mentions that a vaccine against aggregates are actually in the works. With all of this tow, Dettmer et al.’s findings are sure to make a big impact in the way future research. References Dettmer, U., Newman, A., Von Saucken, V., Bartels, T., & Selkoe, D. (2015). KTKEG repeat motifs are key mediators of normal α-synuclein tetramerization: Their mutation causes excess monomers and neurotoxicity. PNAS, 112(31), 9595-9601. doi:10.1073/pnas.1505953112 Kara E, Lewis PA, Ling H, Proukakis C, Houlden H, Hardy J. α-Synuclein mutations cluster around a putative protein loop. Neuroscience Letters. 2013; 546:67-70. doi:10.1016/j.neulet.2013.04.058. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3694303/ National Library of Medicine (US). Genetics Home Reference [Internet]. Bethesda (MD): The Library; 2015 Sep 28. SNCA Gene; [reviewed 2012 May; cited 2015 Sep 29]. Available from: http://ghr.nlm.nih.gov/gene/SNCA Reynaud, E. (2010) Protein Misfolding and Degenerative Diseases. Nature Education 3(9):28. Available from: http://www.nature.com/scitable/topicpage/protein-misfolding-anddegenerative-diseases-14434929 University of California - San Francisco. (2015, August 31). New type of prion may cause, transmit neurodegeneration: Multiple System Atrophy is described as first new human prion disease identified in 50 years. ScienceDaily. Retrieved September 29, 2015 from www.sciencedaily.com/releases/2015/08/150831163735.htm