Calculating Percent Change in Mass for the Potato Lab
advertisement

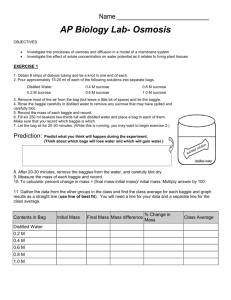
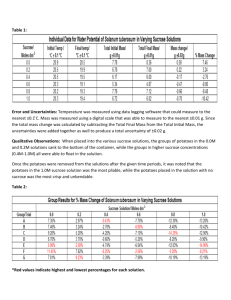
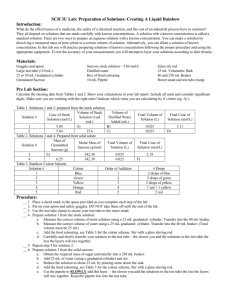
Name__________________________________________________ Date_______________ Period___________ Potato Lab Question: How does increasing the concentration of sugar in water effect the mass of potato cubes? Hypothesis: If…..________________________________________________________ ____________________________________________________________ then…______________________________________________________ ____________________________________________________________ because….___________________________________________________ ____________________________________________________________ The Experiment: Manipulated Variable: ___________________________________________________ Controlled Variables: ____________________________________________________ ______________________________________________________________________ _____________________________________________________________________ Directions 1. Label 4 small beakers with your Period, Group #, and the concentration of sucrose in each jar. Period ___ Group ___ Period ___ Group ___ Period ___ Group ___ Period ___ Group ___ 0% Sucrose 7% Sucrose 14% Sucrose 21 %Sucrose 2. Add 75 mL of the appropriate sucrose solution to each container. 3. Obtain 4 potato cubes from your teacher. 4. Use a triple beam balance to find the initial mass for each potato cube. Once you find the mass of the potato cube, record the data in your data table and place the potato cube in its appropriate container. 5. Next class period, remove each potato cube from its container, dry it off and record the final mass in your data table. 6. Calculate the percent change in mass for each potato cube and share your data with the class. 1 Calculating Percent Change in Mass for the Potato Lab 1. First, dry off each potato cube with a paper towel. 2. Find the “Final Mass” of each potato cube and write their masses in your data table. 3. To find the “Mass Difference,” subtract the initial mass from the final mass. Mass Difference= Final Mass- Initial Mass 4. To find the “Percent Change in Mass,” divide the “Mass Difference” by the “Initial Mass.” Percent Change in Mass= Mass Difference Initial Mass Example: Initial Mass= 3.2 g Final Mass = 3.4 g Mass Difference= 3.4g – 3.2g = .2g Percent Change in Mass = .2g= .06= 6% increase in mass 3.2g Practice: Initial Mass= 3.6 g Final Mass = 3.2 g Mass Difference= _________– ________ = _____________ Percent Change in Mass = __________= ___________= ____________% Data Table *PLEASE SHOW YOUR WORK Concent Initial Final Mass -ration Mass of Sugar 0% Mass Difference Percent Change in Mass 7% 14% 21% 2 Analysis Questions for the Potato Lab *Please answer these questions. Use complete sentences. Attach your answers to your conclusion. 1. How is a cell membrane like a screen door? 2. What is the difference between passive transport and active transport through a cell membrane? 3. Which molecules can diffuse through the cell membrane? 4. What happened to the mass of the potato as the sucrose (sugar) concentration increased? 5. Were the results of this experiment consistent with your hypothesis? Why or why not? 6. What do you think caused the results of this experiment? Conclusion Write your conclusion after you have a whole class discussion regarding the results of this experiment. While writing your conclusion, you should use the CONCLUSION TEMPLATE. Be sure to include answers to the following: 1. Summarize the results of your experiment 2. Revisit your hypothesis. Was your hypothesis correct, incorrect or partially correct? How do you know? 3. Describe any errors in your experiment that could have impacted your results. 4. Explain the results of the experiment. Why did molecules move the way that they did? 5. Apply this phenomenon to a situation out of this class. How does this concept relate to your life? 3 Teacher Set Up The molar mass of sucrose is 342.3 g/mol. Russet potatoes have a sucrose concentration of approximately .2mol/L. As they are placed in a more dilute solution, their mass increases. As the molarity of sucrose increases, the water diffuses out of the potatoes and their mass decreases. Instead of teaching students molarity during 7th grade, I translated these values to percent sucrose. Directions Set up (4) 1 Liter beakers as follows: 1. Add 1 Liter of water to all four beakers 2. Dissolve 68.4 g of sucrose in one beaker (approximately 7% sucrose beaker). 3. Dissolve 136.9 g of sucrose in another beaker (approximately 14% sucrose beaker). 4. Dissolve 205.3 g of sucrose in a third beaker (approximately 21% sucrose solution). 5. Leave the fourth beaker as it is (0% sucrose solution). Before each class, chop up approximately 1 cubic centimeter cubes of russet potatoes. Each group will need 4 potatoes for the experiment. 4