Zooplankton - Jackson Wyers
advertisement

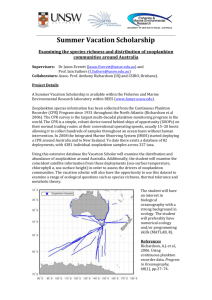
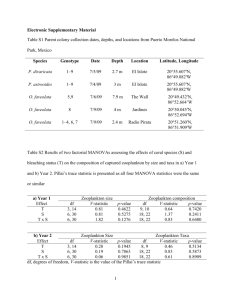
Jackson Wyers Nathan D. Johnson Field Trip Report MARB 435 Identifying and Collecting the Zooplankton of Galveston Introduction Zooplankton are heterotrophic organisms that are suspended in the water column at a variety of depths. The most common zooplankton caught mesozooplankton which includes groups such as copepods. The term mesozooplankton is a size defining term – meaning, it encompasses zooplankton from the 200µm range to 2mm range. These animals are important because they serve as a vital food source to animals higher up in the food chain. On January 28th 2013 a field trip was conducted to collect plankton from the Galveston ship channel. Besides using different collection methods, the main purpose of this experiment was to be able to identify the local species of zooplankton down to species. Identification of planktonic species is important because it reveals a vast amount of information about other marine life in the area – ie: larger marine animals that feed of these mesozooplankton. Water measurements were also taken to check the environmental parameters that the zooplankton were living in. Materials and Methods The location that was selected for this specific experiment was a fishing pier that extends approximately 20 feet out into the ship channel. Before collection, YSI data was recorded for the current water conditions. To collect specimens, two different setups were used. One plankton tow net was weighted so that it would sink; the other net was not weighted so that it would skim the surface of the water. The mesh net that was weighted was held so that it stayed approximately three feet just underneath the surface. Students were divided up into two groups to work the two different nets. Each net was slowly walked down the pier and back – for approximately 30 seconds each time – and then the collection was emptied into a collection bucket – which was prefilled with a little bit of seawater, but not too much so that the samples were in a concentrated manner. The net was then handed off to the next peer in the group and the process was repeated for both groups. Overall, the process was conducted about five to six times with students emptying the nets into the bucket each time. Once the process was done, the equipment and buckets were collected and returned to the lab for analysis. Samples of both deep and shallow water were collected in a small dish and observed under a microscope. Dissecting scopes were used for a broad identification of the organism. For a closer examination, a few drops of Protoslo solution were added to induce a more docile state to the animal; the organism was then pipetted out and onto a slide for further examination under a compound microscope. Results Of the zooplankton identified, the most common was the copepod Acartia tonsa which was found at both depths. The species that were observed from the deeper water overall were as follows according to Table 1: Table 1. Shown below are the species caught and identified from both tows. Deep Water Tow (~3ft.) Surface Tow Chaetoceros lorenzienus Mnemiopsis sp Acartia tonsa Acartia tonsa Seba ekepuu Balanus venustus Temora turbinata Barnacle cyprid Balanus, Caligas Pselicnema Beroe ovata Whenever an organism could not be identified with the dissecting microscope, it was collected onto a slide and then examined with a light microscope for further detail and magnification. The water readings that were recorded were as follows according to Table 2: Table 2: YSI data for location of expeiment. Water Temperature ºC pH Salinity 15.9 7.29 29.8 Dissolved O2 (ppm) 90.3 One of the larger species that was caught was Beroe ovate. This animal was a little more than 1mm in length on average. Figure 1 may be referenced for a visualization of physical characteristics. Discussion The species that were observed and reported would most likely coincide with the environment at the time. Considering this region of Northern America, the water is fairly cold at the moment. According to Jonathan Bird (1999) – author of Plankton: Ocean Drifters – a combination of winter disturbances in the weather and the disappearance of the thermocline are what allow for more nutrients to be present at the surface of the water. This is an interesting point to consider when looking at the data received. With this notion, one might expect there to be a higher abundance of the zooplankton on or near the surface instead of several feet below. However, the data would reveal just the opposite; there was a greater presence of zooplankton from the deeper water sample. Perhaps the water just is not cold enough and this hypothesis therefore is not valid. This is a good possibility when comparing Galveston to other climates around the globe. A more likely explanation is that a higher abundance of zooplankton were found deeper because the experiment was run around mid-afternoon; and because of diel vertical migration patterns, there would be less plankton on the surface as compared to other depths. It was observed that the copepod Acartia tonsa was by far the most abundant species recorded from the samples. This was an expected result since copepods often represent up to 80% of the catch. Another common species was the Barnacle Figure 1: Scientific drawing of species Beroe ovata cyprid. This was also a common organism. One possibility is that the Barnacle populations may have been thriving if more nutrients have been available due to winter disturbances. This species was particularly hard to view under the dissecting microscope because of its speed. To view this plankton with precision, a slide was prepared from the sample that was under the dissecting microscope, and was put under a compound scope. It was not a surprise to find Mnemiopsis sp. since several specimens of Beroe ovata were identified. B. ovata is a predator of Mnemiopsis sp. so it was expected to find this particular organism. Taxon Description Kingdom: Animalia Phylum: Ctenophora Class: Nuda Order: Beroida Family: Beroidae Genus: Beroe Species: Ovata Beroe ovata is a marine zooplankton that is considered nekton because of its ability to move freely in the water column. Their habitat is highly diverse with wide ranges of salinity measures and regions. They are typically pelagic ranging organisms but can also be found in near-shore environments ("Ecosystems where Beroe ovata occurs", 2010). B. ovata is bi-radially symmetrical and have a tapered oval shape with the oral end being the widest part. They are a transparent light color with a hint of pink (Finenko, et al., 2003; Shiganova, et al., 2001). Little is known about the reproductive strategies of this species however they are classified as hermaphrodites and each organism will discharge the egg or sperm into the water where they will float freely. Some other reproductive qualities include year-round breeding times and group spawning (Mills, 2001). These qualities are a synapomorphie of all ctenophores. The preferred prey choice of B. ovata is mostly other ctenophore organisms. A very common feeding choice is that of Mnemiopsis leidyi. Beroe ovata will expand the oral cavity to create negative pressure which will inhale the target prey (Matsumoto & Harbison, 1991; Swanberg, 1974). Some natural significance of these organisms is their ability to control the populations of unwanted animals because of their status as a predator. They do not present any harm to humans due to the lack of stinging tentacles. All in all, the organism has little to no effect on humans (Kube, et al., 2007). References 2010. "Ecosystems where Beroe ovata occurs" (On-line). SeaLifeBase. http://www.sealifebase.org/trophiceco/EcosysList.php?ID=87891&GenusName=Beroe& SpeciesName=ovata. Bird, J. (1999). Plankton: Ocean drifters. Retrieved from http://www.oceanicresearch.org/education/films/planktonscript.htm Finenko, G., Z. Romanova, G. Abolmasova, B. Anninsky, L. Svetlichny, E. Hubavera, L. Bat, A. Kidneys. 2003. Population dynamics, ingestion, growth and reproduction rates of the invader Beroe ovata and its impact on plankton community in Sevastopol Bay, the Black Sea. Journal of Plankton Research, 25 (5): 539-549. http://plankt.oxfordjournals.org/content/25/5/539.full. Kube, S., L. Postel, C. Honnef, C. Augustin. 2007. Mnemiopsis leidyi in the Baltic Sea – distribution and overwintering between autumn 2006 and spring 2007. Aquatic Invasions, 2 (2): 137-145. Matsumoto, G., G. Harbison. 1991. In situ observations of foraging, feeding, and escape behavior in three orders of oceanic ctenophores: Lobata, Cestida, and Beroida. Marine Biology, 117 (2): 279-287. http://www.springerlink.com/content/q9245n0282972844/. Mills, C. 2001. "Ctenophores" (On-line). http://faculty.washington.edu/cemills/Ctenophores.html. Shiganova, T., Y. Bulgakova, S. Volovik, Z. Mirzoyan, S. Dudkin. 2001. The new invader Beroe ovata Mayer 1912 and its effect on the ecosystem in the northeastern Black Sea. Hydrobiologia, 451 (1-3): 187-197. http://www.springerlink.com/content/u417x62p70646858/. Swanberg, N. 1974. The feeding behavior of Beroe ovata. Marine Biology, 24 (1): 69-76. http://www.springerlink.com/content/x348516010343p0t/.