Investigative Science Mystery Powders Lab
advertisement

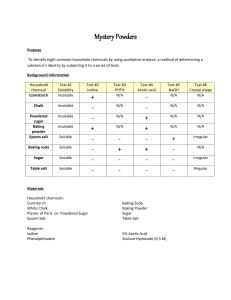
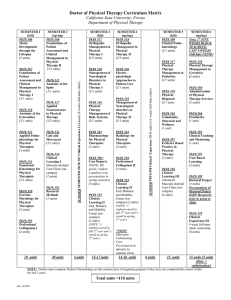
Mystery Powders Purpose To identify eight common household chemicals by using qualitative analysis, a method of determining a substance’s identity by subjecting it to a series of tests. Background Information Household chemical Test #2 Iodine Cornstarch Test #1 Solubility in water Insoluble Test #3 PHTH Test #4 Acetic acid Test #5 NaOH + - + - - Chalk Insoluble - - - - - Powdered sugar Baking powder Epsom salt Insoluble - - + - - Insoluble + - + - - Soluble - - - + - Baking soda Soluble - + - - - Sugar Soluble - - - - Rectangle w/ pointed ends Table salt Soluble - - - - Cube-shaped Materials Household chemicals: Cornstarch White Chalk Plaster of Paris -or- Powdered Sugar Epsom Salt Baking Soda Baking Powder Sugar Table Salt Reagents: Iodine Phenolphthalein (PHTH) 5% Acetic Acid Sodium Hydroxide (NaOH) (0.3 M) Test #6 Crystal shape Safety You will be working with unknown chemicals. Handle them carefully and never, ever taste them! If you are unsure about any procedure, ask your instructor. Avoid spillage. Use small quantities, no more than is required for each test. Never place unused chemicals back in their original container; doing so can contaminate the stock material. Consult your instructor for proper disposal. Procedure: You will be given 8 vials of different kinds of white powder. Your task is to identify these unknowns based on their different physical and chemical properties. Tests: 1. Solubility in Water Place a pea-sized amount of the unknown solid in a test tube and add 5 mL of water. Swirl the test tube to mix the contents. If it dissolves completely, there will be no crystals or powder left in the mixture. Record what happens to each household chemical in Table 2. 2. Tincture of Iodine NOTE! Test the unknown household chemicals that were insoluble in water only. Place a peasized amount of each household chemical in a separate well in a spot plate. Add 2 drops of tincture of iodine to the unknown. A deep blue color forms if the substance contains starch. 3. Phenolphthalein (PHTH) NOTE! Test the unknown household chemicals that were soluble in water only. Place 10 drops of each dissolved unknown into a separate well of the spot plate. Add a drop of phenolphthalein (PHTH) to the dissolved unknown and a bright pink color will result if the solution is alkaline (pH >7). 4. Acetic Acid 5% Test all unknown household chemicals. Place a pea-sized amount of each household chemical in a separate well in a spot plate. Add 2 drops of acetic acid to the unknown. The formation of bubbles is a sign of the carbonate ion, which decomposes to gaseous carbon dioxide upon treatment with an acid. 5. Sodium Hydroxide (0.3 M) NOTE! Test the unknown household chemicals that were soluble in water only. Place 10 drops of each dissolved unknown into separate wells of the spot plate. Place 5 drops of NaOH onto the dissolved unknown. A solid precipitate will form when the reagent is added if the unknown contains magnesium sulfate. 6. Crystal Shape NOTE! Test the unknown household chemicals that were soluble in water only. Place a peasized amount of each household chemical on a watch glass. Use a clean spatula to separate the individual crystals. Use a hand lens (or dissecting microscope) to observe the shape of the crystals for each unknown chemical. INVESTIGATIVE SCIENCE LAB: MYSTERY POWDERS Data Unknown number Test #1 Solubility Test #2 Iodine Name_____________________ Prd____Date_______________ Test #3 PHTH Test #4 Acetic acid Test #5 NaOH Test #6 Crystal shape #1 #2 #3 #4 #5 #6 #7 #8 Analysis and Conclusions 1. Identify your unknowns. Unknown #1 ________________________ Unknown #5 ________________________ Unknown #2 ________________________ Unknown #6 ________________________ Unknown #3 ________________________ Unknown #7 ________________________ Unknown #4 ________________________ Unknown #8 ________________________ 2. Which of the tests used in this experiment measure physical properties and which measure chemical properties? Be sure to identify all 6 tests in your answer. ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 3. Create a qualitative analysis scheme to show how the chemicals can be systematically identified. This should be a flow chart that someone could follow to determine the identity of these 8 chemicals. (HINT: Which test should be done first, second, etc.) Use the back of this page to show your flow chart →