Mystery Powders Lab

Mystery Powders
Purpose
To identify eight common household chemicals by using qualitative analysis, a method of determining a substance’s identity by subjecting it to a series of tests.
Background Information
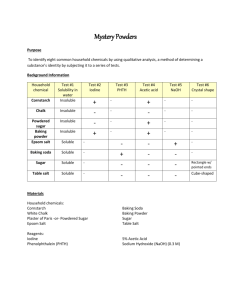
Test #3
PHTH
N/A
Test #5
NaOH
N/A
Test #6
Crystal shape
N/A
Household chemical
Cornstarch
Chalk
Powdered sugar
Baking powder
Epsom salt
Baking soda
Test #1
Solubility
Insoluble
Insoluble
Insoluble
Insoluble
Soluble
Soluble
Sugar
Table salt
Soluble
Soluble
Materials
Household chemicals:
Cornstarch
White Chalk
Plaster of Paris -or- Powdered Sugar
Epsom Salt
Reagents:
Iodine
Phenolphthalein
Test #2
Iodine
+
-
-
+
-
-
-
-
N/A
N/A
N/A
-
+
-
-
Test #4
Acetic acid
-
-
+
+
-
+
-
-
Baking Soda
Baking Powder
Sugar
Table Salt
5% Acetic Acid
Sodium Hydroxide (0.3 M)
N/A
N/A
N/A
+
-
-
-
N/A
N/A
N/A
Irregular
N/A
Irregular
Regular
Safety
You will be working with unknown chemicals. Handle them carefully and never, ever taste them! If you are unsure about any procedure, ask your instructor. Avoid spillage. Use small quantities, no more than is required for each test. Never place unused chemicals back in their original container; doing so can contaminate the stock material. Consult your instructor for proper disposal.
Procedure
Before you begin the first test, make sure all of the test tubes are right side up and numbered (1-8).
Tests:
1.
Solubility in Water
Place a pea-sized amount of each unknown solid in its corresponding test tube and add 5 mL of distilled water. Swirl the test tube to mix the contents. Add an additional 5 mL of water and swirl again. Add another 5 mL of water. Record the behavior of each household chemical in
Table 2. If the substance dissolves write “soluble.” If the substance does NOT dissolve write
“insoluble.”
2.
Iodine
Test all unknown household chemicals. Place a pea-sized amount of each household chemical in a separate well in a spot plate. Add 1 drop of tincture of iodine to the unknown. A deep blue/black color forms if the substance contains starch or a complex carbohydrate (+ reaction).
NOTE! Carefully clean off the spot plate with soap under running water. Dry completely.
3.
Phenolphthalein
NOTE! Test the unknown household chemicals that were soluble in water only. Place 10 drops of each dissolved unknown (from the test tube) into a separate well of the spot plate.
Add a drop of phenolphthalein (PHTH) to the dissolved unknown. A bright pink color (+result) will result if the solution is alkaline (pH > 7).
NOTE! Carefully clean off the spot plate with soap under running water. Dry completely.
4.
Acetic Acid 5%
Test all unknown household chemicals. Place a pea-sized amount of each household chemical in a separate well in a spot plate. Add 2 drops of acetic acid to the unknown. The formation of bubbles (+ result) is a sign of the carbonate ion, which decomposes to gaseous carbon dioxide upon treatment with an acid.
NOTE! Carefully clean off the spot plate with soap under running water. Dry completely.
5.
Sodium Hydroxide (NaOH)
NOTE! Test the unknown household chemicals that were soluble in water only. You will NOT be using the spot plate for this test. Instead, place 5 drops of NaOH directly into the test tube that contains the dissolved unknown. A solid precipitate will form (+ reaction) when the reagent is added if the unknown contains magnesium sulfate.
6.
Crystal Shape
NOTE! Test the crystalline (not powder!) unknown household chemicals that were soluble in
water only. Place the smallest amount you can pinch between your fingers of each household chemical onto the black lab table. Separate the individual crystals. Use a hand lens to observe the shape of the crystals for each unknown chemical (results will be irregularly shaped crystals
or regularly shaped crystals.)
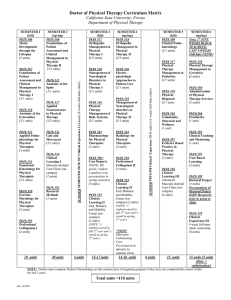
Data
Unknown number
Test #1
Solubility
#1
#2
#3
#4
#5
#6
#7
#8
Analysis and Conclusions
1.
Identify your unknowns.
Test #2
Iodine
Unknown #1 ________________________
Test #3
PHTH
Test #4
Acetic acid
Test #5
NaOH
Test #6
Crystal shape
Unknown #5 ________________________
Unknown #2 ________________________
Unknown #3 ________________________
Unknown #6 ________________________
Unknown #7 ________________________
Unknown #4 ________________________ Unknown #8 ________________________
2.
Which of the tests used in this experiment measure physical properties and which measure chemical properties?
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
______________________________________________________________________________
3.
Create a qualitative analysis scheme to show how the chemicals can be systematically identified.