Master Vocabulary List

BIOLOGY CHAPTER 1 VOCABULARY
Scientific Theories have a lot of data to support them from different branches but are not definitive, or often can never be definitive, they are in essence hypotheses with lots of supportive evidence
Scientific Laws have no evidence that disputes or calls into question their validity or predictive accurac
The Steps of the Scientific Method
Observe a phenomenon and pose a question.“What is the relationship between X and Y”?
Formulate a Hypothesis (an answer your question)-“I think that the relation ship between X & Y (direct/indirect relationship)”
Test Hypothesis -Most often we survey existent data (do research) but we can also…
Experiment -A Good Experimental design isolates variables using strictly defined parameters
The
The
Controlled or Dependent Variable-is the thing you change
Uncontrolled or Independent Variable- is the thing that changes in response
Control Setup = Experimental Setup – Controlled Variable
Data Collection and Analysis -
We collect and display datea using Tables & Graphs,
We analyze data using Statistical instruments like mean median, mode
Conclusion -
A conclusion is a concise explanation as to whether your data supports or does not support your hypothesis
Share your work- Scientists publish results and make sure they are replicable
The Metric System- is based on root 10 or decimal system
Important Suffixes to know
Kilo (k) = 1000
Deci (d ) = 1/10
Centi (c) = 1/100
Milli (m)= 1/1000
Nano (n) = 1/1000000000
BiologyIS the study of life
We measure the following quantities
Mass = grams g
Length = meter m
Temperature = o Celsius or Centigrade o C
Volume solid = meter 3 Volume liquid = liter l
Other Branches of Biology include...(this is a short, very incomplete list)
Biochemistry - macromolecules like proteins, lipid, carbohydrates, and nucleic acids
Cytology - the study of cells, cell types , their structures and metabolism
Genetics - the study of inherited traits
Microbiology
– the study of unicellular organism, archaebacteria eubacteria, protists and fungi
Evolution - the study of the change in species over time
Botany - plants
Zoology
– animals
Ecology
– the relationship between living and nonliving
The Characteristics of Living things (According to this book) CHHRRGM (CHARGEM!!!)
Homeostasis - the maintenance of a stable inte4rnal environment
Metabolism - the cycling of matter and energy Reproduction - asexual v. sexual
Cells - uni v. multi, and prokaryotic v. eukaryotic
Heredity
– pass traits form parent to offspring via DNA
Response - homeostasis, irritability, evolution
Growth and Development- cell division and cell differentiation
CHAPTER 18 TAXONOMY VOCABULARY
CARROLL LINNAEUS –the father of taxonomy
THE 3 DOMAINS –
ARCHAE- CHEMOSYNTHETIC ANCIENT BACTERIA -PROKARYOTE
BACTERIA – MODERN BACTERIA - PROKARYOTE
EUKARYA – EUKARYOTES INCLUDING THE 4 KINGDOMS= PROTISTA-FUNGI-PLANTAE –ANIMALIA
PROTISTA- UNICELL-AUTO AND HETEROTROPHS
FUNGI- MULTI AND U NICELL- HETEROTROPHS
PLANTAE- MULTICELL AUTOTROPHS
ANIMALIA- MULTICELL HETEROTROPHS
THE ORDER OF THE TAXONOMIC CLASSES –KINGDOM- PHYLUM-CLASS-ORDER –FAMILY- GENUS- SPECIES
SCIENTIFIC NAME Genus species Homo sapiens
CHAPTER 3 THE CHEMISTRY OF LIFE VOCABULARY
ATOMS
What makes up matter?
ATOM - matter is made up of atoms. An atom has a positively charged core surrounded by a negatively charged region.
NUCLEUS of an atom is made up of positively charged protons and uncharged neutrons.
ELECTRONS- Negatively charged electrons have little mass and move around the nucleus in a large region called the electron cloud.
Every living and nonliving thing is made of matter. Matter is anything that has mass and takes up space.
An atom is the smallest unit of matter that cannot be broken down by chemical means.
ELEMENT is a substance made up of atoms that have the same number of protons.
PROTON
–
For example, each atom of the element carbon has six protons.
ISOTOPES
–
Atoms of an element may have different numbers of neutrons. These atoms are called isotopes of elements.
IONS – charged atoms that have lost or gained electrons
CHEMICAL BONDS
OCTET RULE
Chemical bonds form because most atoms become stable when they have eight electrons in the valence shell.
VALENCE ELECTRONS Electrons in the outermost level, or shell. Atoms tend to BOND so that eight electrons will be in the valence shell.
CHEMICAL BONDS -When atoms combine, a force called a chemical bond holds them together.
COMPOUND - When atoms of different elements combine, a compound forms. A compound is a made of the bonded atoms of two or more elements.
COVALENT BOND -One way that atoms bond is by sharing valence electrons to form a covalent bond.
MOLECULE
– a group of atoms held together by covalent bonds.
– A water molecule, H2O, oxygen atom covalent bonds with two hydrogen atoms.
IONIC BONDS - Atoms can achieve a stable valence level by losing or gaining electrons , resulting in a positive or negative charge.
ION an atom that has an electric charge because it has gained or lost electrons.
–
The attractive force between oppositely charged ions is an ionic bond.
POLARITY - Molecules with partial charges on opposite ends are said to be polar.
WATER IS POLARThe partially charged ends of polar molecules attract opposite charges. Because of this behavior, polar molecules can dissolve other polar molecules and ionic compounds, Water can dissolve sugar and salt. Nonpolar substances, such as oil, grease, and wax, do not dissolve well
PROPERTIES OF WATER Most of the unique properties of water result because water molecules form hydrogen bonds with each other.
ICE IS LESS DENSE = FLOATS - the crystal structure formed due to hydrogen bonding makes ice less dense than liquid water.
LATENT HEAT - Water can absorb a large amount of heat without changing temperature. -helps organisms maintain a constant internal temperature.
COHESION- The attraction of particles of water . Cohesion keeps water from evaporating easily- water is a liquid at ordinary temperatures.
ADHESION- Water molecules also stick to other polar molecules.
SOLUTIONS- A solution is a mixture in which ions or molecules of one or more substances are evenly distributed in another substance.
DISSOCIATION OF WATER water molecules break apart to form H+ and hydroxide ions in equal numbers.= pH 7
–
Acids are compounds that form extra H+ ions when dissolved in water.
–
Bases are compounds that form extra OH- ions when dissolved in water. pH is a measure of how acidic or basic a solution is.
–
Each one-point increase in pH represents a 10-fold decrease in hydronium ion concentration.
–
Pure water has a pH of 7. Acidic solutions have a pH below 7, and basic solutions have a pH above 7.
–
The pH of solutions in living things must be stable. For a stable pH the solutions in living things contain buffers.
–
A BUFFER is a substance that reacts to prevent pH changes in a solution.
CARBON Compounds BUILDING BLOCKS OF CELLS
POLYMERS Large, complex biomolecules are built from a few smaller, simpler, repeating units arranged in an precise way.
BIOMOLECULES The parts of a cell are made up of large, complex molecules,
CARBON COMPOUNDS = ORGANIC COMPOUNDS- biomolecules contain carbon. Carbon atoms form covalent bonds with as many as four atoms.
CARBOHYDRATES Cells use carbohydrates for sources of energy , structural materials, and cellular identification.
Carbohydrates are molecules made of sugars. A sugar contains carbon, hydrogen, and oxygen in a ratio of 1:2:1.
Carbohydrates are a major source of energy for many organisms, including humans. Chitin is found in the shells of insects
and the cell walls of mushrooms.
– Cellulose is found in the cell walls of plants.
LIPIDS –POLYMERS OF FATTY ACIDS The main functions of lipids include storing energy and controlling water movement.
Lipids includes fats, phospholipids, steroids, and waxes. consist of chains of FATTY ACIDS
ENERGY STORAGE- The main purpose of fats is to store energy. Fats can store energy even more efficiently than carbohydrates.
PHOSPHOLIPIDS - CELL MEMBRANE is made of phospholipids. The structure of membranes depends on how this molecule interacts with water.
Waxes, found on the surfaces of plants and aquatic bird feathers, help prevent evaporation of water from the cells of the organism.
PROTEINS- POLYMERS OF AMINO ACIDS Proteins are chains of amino acids that twist and fold into certain shapes that determine FUNCTION
Proteins are involved in structure, support, movement, communication, transportation, and carrying out chemical reactions.
A protein is a molecule made up of amino acids, building blocks that link to form proteins.
–
Every amino acid has an amino group and a carboxyl group. Units of amino acids can form links called PEPTIDE
BONDS
For each type of protein, amino acids are arranged in a specific order, the protein’s primary structure.
– The interactions of the various side groups may form coils and folds, the protein’s secondary structure.
– The overall shape of a single chain of amino acids is the protein’s tertiary structure.
–
The quaternary structure is the overall shape that results from combining the chains to form proteins.
NUCLEIC ACIDS – DNA & RNA ARE POLYMERS OF NUCLEOTIDES ATP IS A NUCLEOTIDE TOO!!
Nucleic acids store and transmit hereditary information.A nucleic acid is a long chain of nucleotide units.
A nucleotide is a molecule made up of three parts: a sugar, a base, and a phosphate group.
–
Nucleotides of deoxyribonucleic acid, or DNA, contain the sugar deoxyribose.
– Nucleotides of ribonucleic acid, or RNA, contain the sugar ribose. DNA molecules CARRY GENES act as “CODES FOR PROTEIN
– DNA consists of two strands of nucleotides that spiral around each other. – RNA also interacts with
DNA to help decode the information.
ATP – Energy is released in the reaction that breaks off the third phosphate group.
Other single nucleotides transfer electrons or hydrogen atoms for other life processes.
–
Adenosine triphosphate, or ATP, is a nucleotide that has three phosphate groups and supplies energy to cells.
Section 4: Energy and Metabolism CHANGING MATTER
Matter is neither created nor destroyed in any change. This observation is called the law of conservation of mass.
Energy may change from one form to another, but the total amount of energy does not change. This is called the law of
conservation of energy.
CHEMICAL REACTIONS
Chemical reactions can only occur when the activation energy is available and the correct
atoms are aligned.
During this process, bonds between atoms are broken, and new ones are formed.
REACTANT is a substance that is changed in a chemical reaction.
PRODUCT is a new substance that is formed.
CHEMICAL REACTION can only occur under the right conditions. To form new bonds, the particles must collide fast enough to overcome the repulsion between their negatively charged electron clouds.
ACTIVATION ENERGYof a reaction is the minimum kinetic energy required to start a chemical reaction. When the reactant particles collide, the correct atoms must be brought close together in the proper orientation.
BIOLOGICAL REACTIONS
ENZYMES -By assisting in necessary biochemical reactions, enzymes help organisms maintain homeostasis.
In living things, chemical reactions occur between large, complex biomolecules. Many of these reactions require large activation energies.
An enzyme is a molecule that increases the speed of biochemical reactions.
–
Enzymes hold molecules close together and in the correct orientation.
- An enzyme lowers the activation energy of a reaction.
–
Each enzyme has an active site, the region where the reaction takes place.
–
The shape of the active site determines which reactants, or substrates, will bind to it. Each different enzyme acts only on specific substrates.
–
Binding of the substrates causes the enzyme’s shape to change. This change causes bonds in the substrates to break and new bonds to form.
– Most enzymes need a certain range of temperatures and pH.
Cells get most of the energy needed for metabolism by breaking down food molecules.
The release of energy from food molecules occurs in a series of reactions using many enzymes to capture energy in the form of ATP molecules.
The enzymes reduce the activation energy so much that only a little energy is needed to start the reactions. In this process, very little energy is lost as heat.
CHAPTERS 4-5-6 ECOLOGY VOCABULARY
ECOLOGY Living things do not live in vacuums, their daily lives are based on interactions with both living BIOTIC and nonliving ABIOTIC components.
BIOSPHERE- all the areas of the earth occupied by organisms
BIOME- a specific area of the biosphere identified by climate elevation soil type and precipitation
ECOSYSTEM- Groups of populations and their physical environment
POPULATION – a group of organisms of the same species in an identifiable region that interbreed
SPECIES- organisms that can interbreed to produce viable offspring
AUTOTROPHS are producers that produce food through PHOTSYNTHESIS or CHEMOSYNHTESIS
HETEROTROPH are consumers that take in premade food.
Herbivores – animals that eat plants
Carnivores – animals that eat other animals
Omnivores – animals that eat plants and animals
Decomposers - bacteria and fungi, that break down dead organic waste.
Detritus - partially decomposed organic matter in the soil and water; beetles, earthworms, and termites are detritus feeders.
Consumers
Consumer Levels
Primary consumer – an organism that gets its energy from plants (producers)
Secondary consumer – an organism that gets its energy from primary consumers
Tertiary_ consumer – carnivores that eat other carnivores; a top-level consumer, usually the top predator in the food chain
ENERGY FLOW- The movement of energy through the organisms in ecosystem- Sun Producers consumers
As energy flows from autotrophs to heterotrophs MOST is lost as HEAT before the consumer can use it.
FOOD CHAIN - diagram that links organisms together by who eats whom
Starts with _plant life_ and ends with an animal_.Most food chains have no more than _4 or 5_ links
Arrows show the direction energy is flowing_EXAMPLE: grass
zebra
lion
FOOD WEB- Most consumers feed on and are eaten by more than one other organsim
A combination of several food chains showing all of the possible energy pathways
TROPHIC LEVEL All of the organisms that feed at a particular link of the food chain/web
Grazing food web – The upper portion of a food web based on a living plant as the FOUNDATION
Detrital food web –The lower portion of a food web based on detritus
ECOLOGICAL PYRAMIDS- Only about 10% of energy is useable from one trophic level to the next
NUMBERS- The number organisms drastically DECREASE as you go up in level of a food chain
A series of blocks representing the biomass of particular organisms on a particular trophic level
BIOMASS -The amount of living material in the population of an organism
Biochemical cycles- The path by which important nutrients/molecules travel through an ecosystem.
3 Important Cycles: Water Cycle, Carbon Cycle, Nitrogen Cycle
The Water Cycle
Water movement:
Land
Atmosphere:Liquid
Gas
EVAPORATION from rivers, lakes and oceans
TRANSPIRATION from plant
Atmosphere
Land:Gas
Liquid
PRECIPITATION over land and bodies of water
RUNOFF forms bodies of water (lakes, rivers, oceans)
Ground water
SEEPAGE/INFILTRATION/PERCOLATION into aquifer
The Carbon Cycle CARBON MOVEMENT
Land/Water
Atmosphere
RESPIRATION
COMBUSTION
Atmosphere
Land/Water
PHOTSYNTHESIS
Dissolved CO
2
** Carbon is stored as fossil fuels from decaying organisms.
The Nitrogen CycleNitrogen Movement:
Nitrogen Fixation
BACTERIA found in legume roots converts N
2
gas into _Ammonia (NH
4
)_
Decomposers_ break down waste and organic remains into _Ammonia (NH
4
)_
Nitrification
bacteria convert ammonia into _Nitrite (NO
2
) and _Nitrate (NO
3
)_ to be used by plants
Denitrification Bacteria converts _ammonia_ back into Nitrogen gas (N
2
)
CHAPTER 7 CELL STRUCTURE AND FUNCTION VOCABULARY
The Cell Theory
Robert Hooke Observed cork (called them cells)
The Cell Theory
1 All organisms composed of one or more cells
Antoine Van Leeuwenhoek
Matthias Schleiden All plants MADE of separate cells
Limitations to Cell Size single-celled organisms "animalcules" 2. Cell is smallest living organizational unit
3. Cells arise only from division of other cells
Faster passage through small cells
More efficient communication
Increase size, greater increase in volume -surface area
Interaction with outside occurs only at surface
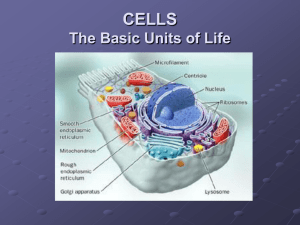
THE STRUCTURE OF EUKARYOTIC CELLS: AN OVERVIEW OF CELL STRUCTURE
The Plasma Membrane Surrounds the Cell- Phospholipid bilayer contains embedded protein
The Central Portion of the Cell Contains the Genetic Material
Genetic material in prokaryotes-Single, circular molecule of DNA
Genetic material in eukaryotes- Contained within the nucleus- Surrounded by two membranes
The Cytoplasm Comprises the Rest of the Cell's Interior- Cytoplasm is a semifluid matrix
Eukaryotes Are More Complex Than Prokaryotes
Key to organization is compartmentalization- Possess internal membrane-bound organelles
Mitochondria and chloroplasts associated with cellular energy
Central vacuole in plants stores protein and wastes
Vesicles in animals store and transport many materials
Cytoskeleton is an internal scaffold of proteins
Cell walls: cellulose/chitin fibers embedded in polysaccharides, proteins
THE ENDOPLASMIC RETICULUM : COMPARTMENTALIZATION OF THE CELL
General Characteristics- Thin membranes not visible in light microscope
Divide interior into compartments
Translate RNA copies of genes into proteins
Exported proteins contain signal sequences
Lipid bilayer with embedded proteins
Rough ER: Manufacturer of Proteins for Export
Ribosomes assist manufacture of proteins
Aggregates of protein and RNA
THE NUCLEUS : INFORMATION CENTER FOR THE CELL
Smooth ER: Organizer of Internal Activities
Lack ribosomes
Contain embedded enzymes
Detoxification, carbohydrate and lipid synthesis
The Nuclear Envelope- Double layer of membranes, outer continuous with ER
The Chromosomes of Eukaryotes Are Complex Proteins Are Synthesized on the Ribosomes
Contain hereditary information
Divided into chromosomes associated with histone protein
Read mRNA to direct synthesis of protein
The Nucleolus - Location of ribosome synthesis
Dark-staining region visible in protein producing cells
THE GOLGI APPARATUS : THE DELIVERY SYSTEM OF THE CELL Function in Molecule Collection, Packaging, Distribution
Golgi Bodies- flattened stacks of membranes-Abundant in glandular secretory cells- called the Golgi complex or apparatus
LYSOSOMES : PRODUCERS OF DIGESTIVE ENZYMES FOR THE CELL- Membrane-Bound Organelles Containing Hydrolytic Enzymes
Enzymes catalyze breakdown of macromolecules within cell Eliminate Other Substances Including Whole Cells
Digest worn-out cell components Digest pathogens engulfed by white blood cells
Mitochondria : The Cell's Chemical Furnaces- Possesses own genome
Occur in all organisms
Bounded by double membrane
Chloroplasts : Where Photosynthesis Takes Place
Occur in photosynthetic organisms, plants and algae
Inner membrane is folded into layers called cristae
Divides into inner matrix and outer compartment
Centrioles : Microtubular Assembly Plants
Internal membranes form disk-shaped thylakoids
Photosynthetic pigments on thylakoid surface
Stack of thylakoids called granum
Possess own genome
Present in animal cells, NOT plant cells
Occur in pairs near nuclear envelope, forms the centrosome
Associated with assembly and organization of microtubules
Form basal bodies that anchor flagella and cilia
THE CYTOSKELETON : INTERIOR FRAMEWORK OF THE CELL
Network of Protein Fibers
Provide Mechanical Support for Cell
Actin fibers determine cell shape
Rapid changes in filament length changes cell shape quickly
FLAGELLA AND CILIA : MOTILITY FOR THE CELL
Eukaryotic Flagella9+2 structure of microtubules
Cilia and Centrioles Also Show 9+2 Arrangement
SYMBIOSIS AND THE ORIGIN OF EUKARYOTES
Eukaryotes Have Radically Different Cell Structure
Possess organelles that resemble bacteria, endosymbiont theory
Symbionts Provided Metabolic Advantage to Host
Mitochondria are energy factories
Chloroplasts photosynthesize
Evidence Supporting Theory
Numerous, short projections called cilia
Have functions other than locomotion
Mitochondria and chloroplasts surrounded by double membrane
Mitochondria and bacteria have similar size
Mitochondrial ribosomes resemble bacterial ribosomes
Mitochondria and chloroplast DNA circular like bacteria
Mitochondria divide by simple fission
CHAPTER 8 CELL TRANSPORT
HOMEOSTASIS -Homeostasis is the maintenance of stable internal conditions in a changing environment.
LIPID BILAYER -The phospholipids form a barrier through which only small, nonpolar substances can pass.
The cell membrane is made of phospholipids made of a phosphate “head” and two fatty acid “tails.” head is polar and is attracted to water, fatty acid tails are nonpolar and are repelled by water. the phospholipids form a double layer called the lipid bilayer.
Only certain substances can pass through the lipid bilayer.
Ions and most polar molecules are repelled by the nonpolar interior of the lipid bilayer.
MEMBRANE PROTEINS include cell-surface markers, receptor proteins, enzymes, and transport proteins.
Cell-surface markers act like a name tag, carbohydrates attached to the surface by proteins called glycoproteins.
Receptor proteins enable a cell to sense its surroundings by binding to certain substances outside the cell.
Enzymes in the cell membrane help SPEED biochemical reactions inside the cell by lowering activation energy
Cell Transport - 2 TYPES PASSIVE OR ACTIVE
EQUILIBRIUM In solution, randomly moving molecules fill up a space, as the space is filled evenly equilibrium is reached.
CONCENTRATION The amount of a particular substance in a given volume
CONCENTRATION GRADIENT When one area has a higher concentration than another area does
DIFFUSION The movement of substances down a concentration gradient is called diffusion.
OSMOSIS- the diffusion of water across a membrane
CELL MEMBRANE separates the cytoplasm from the fluid outside the cell made of phospholipids
PASSIVE TRANSPORT - substances cross the cell membrane down their concentration gradient. NO ATP used
–
The direction of movement depends on the concentration gradient and does not require energy.
– Some substances diffuse through the lipid bilayer. Other substances diffuse through transport proteins.
1.SIMPLE DIFFUSION
–
Small, nonpolar molecules can pass directly through the lipid bilayer. Like O2 or CO2
Oxygen moves down its concentration gradient into the cell. Carbon dioxide diffuses out of the cell.
2. CARRIER FACILITATED DIFFUSION - proteins help substances diffuse through the cell membrane.
Two types of transport proteins are channel proteins and carrier proteins.
CHANNEL PROTEINS ions and polar molecules important for cell function diffuse through channel proteins.
TRANSPORT PROTEINS sometimes called pores, serve as tunnels through the lipid bilayer.
3.OSMOSIS
Water can diffuse across a selectively permeable membrane in a process called OSMOSIS.
Osmosis in cells is a form of facilitated diffusion. The direction of water movement depends on the concentration of the environment.
HYPERTONIC SOLUTION has a higher solute concentration than the cytoplasm does, water moves out of the cel
ISOTONIC SOLUTION or has the same solute concentration that the cytoplasm does, water diffuses into and out of the cell at
HYPOTONIC SOLUTION or has a lower solute concentration than the cytoplasm does, water moves into the cell.
The cell gains water and expands in size. If left unchecked, the swelling caused by a hypotonic solution could cause a cell to burst.
ACTIVE TRANSPORT Active transport requires energy to move substances against their concentration gradients.
In order to move substances against their concentration gradients, cells must use energy.ATP.
1. PUMPS -In active transport, the carrier proteins do require energy to “pump” substances against their concentration gradient.
The sodium-potassium pump is a carrier protein that actively transports three sodium ions out of the cell and two potassium ions into the cell.
2.ENDO/EXOCYTOSIS- some materials cross the cell membrane in vesicles, which are membrane-bound sacs.
The movement of a large substance into a cell by means of a vesicle is called endocytosis.
The movement of material out of a cell by means of a vesicle is called exocytosis.
Section 3: Cell Communication
How do cells use signal molecules?
RESPONDING TO SIGNALS - cell may respond to signal by changing membrane permeability, activating enzymes, or by forming a second messenger.
PERMEABILITY CHANGE- Transport proteins may open or close in response to a signal.
ENZYME ACTIVATION- Some receptor proteins are enzymes or they activate enzymes in the cell membrane.
SECOND MESSENGER Binding of a signal molecule outside the cell may cause a second messenger to form. The second messenger acts as a signal molecule within the cell and causes changes in the cytoplasm and nucleus.
CHAPTER 9 CELL RESPIRATION AND PHOTOSYNTHESIS VOCABULARY
LEVEL 1 YOU MUST KNOW THIS !!!
Solar energy in
CO
2
+ H
2
O -------------------- > C
6
H
12
O
6
+ O
2
PHOTOSYNTHESIS - OCCURS IN CHLOROPLAST
C
6
H
12
O
6
+ O
2
-------------------- > CO
2
+ H
2
O CELL RESPIRATION – OCCURS IN MITOCHONDRIA
ATP out
LEVEL 2 YOU SHOULD KNOW THIS TOO!!!!
PHOTOSYNTHESIS 2 REAXNS
Solar energy in
CO
2
+ H
2
O --------------------> C
6
H
12
O
6
+ O
2
LIGHT REACTIONS H
2
O ----> O
2
HAPPENS IN THE THYLAKOID OF THE CHLOROPLAST!!
DARK/CALVIN CO
2
----> C
6
H
12
O
6
C
6
H
12
O
6
IS STORED SOLAR ENERGY!!!
CELL RESPIRATION 5??REAXNS
C
6
H
12
O
6
+ O
2
-------------------- > CO
2
+ H
2
O
ATP out
1. GLYCOLYSIS
2. KREBS TCA CYCLE
C
6
H
12
O
6
----> PYRUVATE ANAEROBIC + 2 ATP
PYRUVATE+ O
2
----> CO
2
+ H
2
O AEROBIC (+ O
2
) + 2 ATP
3. ELECTRON TRANSPORT CHAIN
DON’T WORRY BOUT IT AEROBIC (+ O
2
) + 32-34 ATP TOTAL 36-38??
OR IF NO O
2
IS PRESENT PYRUVATE -----
FERMENTATION 4. LACTIC ACID AND/OR 5. ALCOHOL
ELECTRON TRANSPORT MOLECULES INCLUDE NADH, NADPH, FADH2, ATP
TOGETHER PHOTSYNTHESIS AND CELL RESP MAKE UP THE CARBON CYCLE!
ENZYMES –PROTEINS THAT SPEED CHEMICAL REACTIONS BY LOWERING ACTIVATION ENERGY
THEY CAN BE AFFECTED BY HEAT pH or changes in enzyme or substrate concentration
ACTIVE SITE- SPECIAL REGION OF ENZYME THAT BINDS SUBSTRATE
SUBSTRATE- REACTANT THATIS ACTED UPON
ENZYMES ARE NOT AFFECTED- THEY KEEP BEING REUSED OVER AND OVER AGAIN
CHAPTER 10 -11 MITOSIS AND MEIOSIS VOCABULARY
MITOSIS- CELL DIVISION- for growth, to repair injuries and replace worn out cells
SURFACE AREA/VOLUME ratio- cells undergo mitosis to maintain an ideal ration
CELL CYCLE- G1, S, G2, M, C
G1- GAP1-normal cell growth
S- Synthesis DNA is REPLICATED
G2- GAP2 – celluar organelles are replicated as the cell prepares to divide
INTERPHASE- INCLUDES G1, S, AND G2
MITOSIS- aka NUCLEAR DIVISION includes prophase, metaphase, anaphase and telophase
PROPHASE –nucleus dissolves , chromosomes appear
METAPHASE- mitotic spindle forms, chromosomes align at equatorial plane of cell
ANAPHASE- centrosome pull fibres which cause chromosomes to separate and move towards cell poles
TELOPHASE- nuclear envelope reappears, chromosomes unwind, cell enters cytokinesis
CYTOKINESIS- CELL DIVISION cells separate completely
CHROMOSOME- condensed DNA and histones (proteins) aka CHROMATIN
CENTROMERE –joins sister chromatids
SISTER CHROMATID –homologous chromosomes joined by centromere
CHROMATIN – histones and DNA
HISTONE – a protein DNA coils around to condense
DNA –genetic Nucleic Acid with approximately 23,000 genes
HOMOLOGOUS –the same
AUTOSOMES- chromosomes that do not code for gender, 22 pairs
SEX CHROMOSOMES- chromosomes that determine gender XX female XY male, 1pair
SPINDLE FIBER- composed of cytoskeletal elements like microtubules and filaments- pulls chromosomes apart
CENTROSOME – site of mitotic spindle formation, anchors normally found near nucleus
GENDER- sex male or female
DIPLOID - having 2 copies of each chromosome in humans all somatic cells are diploid with 46 chromosomes
HAPLOID – havnig 1 copy of each chromosome, gametes sperm eggs are Haploid
SPERM - male gamete or germ cell
EGG/OVUM- female gamete or germ cell
PROKARYOTE – single celled anucleate bacterium
BINARY FISSION – the primary method fro prokaryotic cell division, and reproduction-
UNICELLULAR- single celled
SPINDLE(MITOTIC) fibre formation seen in metaphase that aligns chromosomes along equator of cell and pulls chromosomes apart
NUCLEUS- control cent of cell
DAUGHTER CELLS- the product of mitosis
CLEAVAGE FURROW- the barrier that occurs between animal cells undergoing telophase and cytokinesis
CELL PLATE- the barrier that occurs between plant cells undergoing telophase and cytokinesis
ZYGOTE- a fertilized egg
GAMETE- sperm or egg
FERTILIZATION- the joining of sperm and egg
SEXUAL REPRODUCTION –the joining of gametes to produce a genetically unique individual
ASEXUAL REPRODUCTION – the reproduction of organisms to produce offspring with identical genetics
MEIOSIS –gamete formation includes Meiosis I and Meiosis II
TETRAD – the joining of 2 pairs (4 ) homologous chromosomes during prophase I of meiosis
SYNAPSIS – the alignment of homologues during tetrad formation allowing the exchange of chromosome parts
CROSSING OVER- the exchange of chromosome sections between homologous chromosomes during tetrad formation
REPLICATION –the copying of DNA necessary for Mitosis and Meiosis to occur
CONDENSATION the formation of chromatin as they coil to form dense dark bodies or chromosomes
MEIOSIS I & II- are like two cycles of mitosis, one cell becomes 2 and the 2 cells become 4- those 4 cells are HAPLOID GAMETES!!
If you see a I or II with prophase, metaphase, anaphase or telophase it Meiosis! If not its MITOSIS
CHAPTER 12 MENDEL AND HEREDITY VOCABULARY
CLASSICAL GENETICS-the study of the inheritance of traits and their patterns
MODERN GENETICS - the study of the biochemical basis of inheritance
HEREDITY is the passing of traits from parents to offspring
GENETICS is the study of how traits are inherited through the action of alleles
GREGOR MENDEL – “Father of Genetics,” responsible for laws governing the inheritance of traits
SEXUAL REPRODUCTION The production of new organism by combing DNA
Plants normally reproduce 2 ways
SELF POLLINATION-pollen from one plant fertilizes ova of same plant
CROSS POLLINATION-pollen from one plant fertilizes another
SELECTIVE BREEDING- The practice of human intervention in creating specific breeds
PURE BREED or TRUE BREEDING STRAIN
– organism that ALWAYS produces offspring of the same type when bred with a member of its “breed.”
SPECIES –organisms that are so similar that they can reproduce VIABLE offspring
HYBRID – the mix of two true breeding strains- extreme examples include ligers or mules
CHARACTER– An identifiable feature of an organism, like flower color or height
TRAITS– any specific characteristic that can be passed from parents to offspring
EXPRESSION – refers to traits that are observed,
HEREDITY– the passing of traits from parents to offspring
DOMINANT– is always expressed; masks a recessive trait
RECESSIVE– can only be expressed if there are no dominant alleles present
ALLELE– one form (dominant or recessive) of a gene
GAMETE- Sex cells (have ONE form of a gene on their chromosomes)
SOMATIC or Body cells have TWO alleles for a single gene
DOMINANT alleles are represented by a capital letter, are expressed in both Homo and heterozygote
RECESSIVE alleles are represented by a lower case letter, only expressed in homozygote
HOMOZYGOUS- two copies of the same allele
BB (homozygous dominant) bb (homozygous recessive)
HETEROZYGOUS,
Bb Organism with two different alleles
GENOTYPE: the alleles present in the organism, BB, Bb, or bb (THE TYPE OF GENES)
PHENOTYPE: the expression of the genes; observed traits (THE TYPE OF TRAITS)
GENETIC CROSSES- Mating trials between organisms
MONOHYBRID CROSS: cross involving ONE trait, e.g., eye color
DIHYBRID CROSS: cross involving TWO traits, e.g., eye color and hair color
PUNNETT SQUARE – Model to determine offspring’s genotype and phenotype
PARENTAL GENERATION (P1 0r Po) = the parental generation in a breeding experiment
FIRST FILIAL GENERATION (F1) = the first generation of offspring in a breeding experiment
SECOND FILIAL GENERATION (F2) = the second generation of offspring in a breeding experiment
MENDELS LAW OF RANDOM SEGREGATION
Each pair of alleles segregates independently during gamete formation in 50/50 proportions
MENDELS LAW OF INDEPENDENT ASSORTMENT
Traits are not connected in any way to each other, ie pea color and pod color aren’t connected
Mendel is a little wrong on this one
GENETIC CROSS FORMULA –determines the number of possible genotypes in a genetic cross
Formula: 2 n (n = # of heterozygotes)
TEST CROSS – Cross that allows you to deduce Parental Genotype by Breeding a dominant with a recessive
ALTERNATIVE MODES OF INHERITANCE – the dominant recessive pattern is not the only way genes are expressed
INCOMPLETE DOMINANCE - Heterozygotes have BLENDED TRAITS -appearance is between the phenotypes of the two parental varieties.
CODOMINANCE- BOTH ALLELES are expressed in heterozygote BOTH TRAITS are observed
SEX LINKED TRAITS - Traits (genes) located on the sex chromosomes, Many sex-linked traits carried on X chromosome
CHAPTER 13: DNA, RNA, and Proteins VOCABULARY
The Central Dogma of Biology DNA is transcribed into RNA->RNA is translated into Proteins
Griffith - Discovered the transformation of harmless R strain Bacteria by heat killed S bacteria
Avery – Identified DNA as the transformative agent
Hershey & Chase –Studied bacteriophages and proved DNA was the source of hereditary information
James Watson & Francis Crick deduced the structure of DNA
Rosalind Franklin used x ray crystallography to image the structure of DNA
DNA- Deoxyribonucleic Acid
DNA is a polymer of Nucleotides- remember a polymer is like a chain of beads
DNA is made of 2 chains, twisted together and coiled into an alpha double helix
DNA Double Helix is composed of 2 interconnected nucleotide chains
Nucleotide Chains are composed of a Sugar-Phosphate backbone, with bases in the middle
Phosphodiester bonds join nucleotides
Nucleotide contains 1 Nitrogenous base + 1 phosphate group + 1 sugar
Deoxyribose the sugar in DNA
4 different Nucleotides abbreviated as A, T G, or C
The pyrimidines -Adenine Guanine
The purines -Cytosine Thymine
Chargraff’s Rules
- A binds T, G binds C according to
Hydrogen bonds .- join the bases across the helix
MITOSIS Cell division
MEIOSIS gamete formation
DNA Replication- is semi conservative!
Helicase breaks hydrogen bonds in the middle of the strand, creating a replication fork
DNA Polymerase , creates 2 identical DNA molecules
Transcription- DNA copied to RNA, occurs in nucleus
RNA - A nucleic acid that is similar to DNA, Ribose is the sugar, instead of thymine , URACIL , single stranded
Steps of Transcription
RNAPolymerase (RNApol)– Unwinds DNA strand and copies it into RNA
Uracil RNA base replacing Thymine
Messenger RNA (mRNA) DNA transcript or copy moves to the ribosome in the cytoplasm (or on the Rough ER)
Translation (Protein Synthesis) - The Conversion of mRNA to Amino Acids which make up proteins
The Genetic code (ATCG) is translated into a amino acids
CODON- a 3 letter sequence of mRNA that codes for a particular Amino Acid
Amino Acid -the components that make Proteins tRNA (transferRNA) the RNA that carries amino acids to the ribosome
Anti-Codon – the 3 letter sequence of tRNA that corresponds to the mRNA CODON
AUG - the start codon
Translation – conversion of mRNA into a Protein
Initiation - Ribosome attaches to mRNA (each 3 bases is called a codon), tRNA brings anticodon and AA to ribosome
Elongation-
More tRNA’s bring more AA’s to ribosome, AA’s connected together to make polypeptides (by peptide bonds)
Termination- Ribosome reaches a STOP signal on mRNA
Enzymes - the most common type of protein
CHAPTER 14: Genes in Action VOCABULARY
Gene -a segment of DNA whose nucleotide sequence codes for a protein.
Mutation - Changes in the nucleotide sequence of a gene’s DNA
Mutagens cause mutations, include environmental factors ike chemicals, X-rays, and UV light
Genetic Mutations – single or small changes to individual genes DNA sequence
Point mutations include; silent, missense and nonsense
SILENT mutation - the change in the codon results in the same amino acid- UAU
UAC both code for tyrosine
NONSENSE mutation - a codon is changed to a stop codon; protein may be too short to function -UAC
UAG (a stop codon)
MISSENSE mutation - involves the substitution of a different amino acid,
Frameshift mutations may be caused by additions or deletions, they create missense or nonsense mRNA
Genetic Diseases ( aka genetic disorders) like: CF, Duchennes MD, ColorBlindness are caused by point or frameshift mutations
Sickle Cell Anemia : MISSENSE MUTANT -Defective Hemoglobin Gene-You can’t carry oxygen well
Cystic Fibrosis: DELETION MUTANT -Defective Protein is made that creates excess mucus; clogs lungs.
Color Blindness : SEX LINKED - Inablity to distinguish colors (8% of male population)
Hemophilia: SEX LINKED - Inablity of blood to clot
Muscular Dystrophy: SEX LINKED -Loss of muscle that begins in early childhood
Chromosomal Mutations . Large scale change in the gene sequence including deletion, duplication, inversion, translocation
Tetrad formation in Prophase I of Meiosis when Chromosomal changes occur
Chromosomal or Genetic Syndromes – caused by chromosomal mutations, include; Williams, Alagille, or Downs
Chromosomal Deletion : Chromosome loses a segment, ex. Williams Syndrome- Chromosome 7 loses an end piece
Chromosomal Duplication: chromosome segment being REPEATED in the same chromosome, ex inv Dup 15 syndrome
Chromosome Translocation: exchange of chromosomal segments between two, NON-HOMOLOGOUS chromosomes. ex.
Alagille Syndrome
Chromosome Inversion- a segment of a chromosome being turned 180 degrees.
Williams Syndrome - Children have a pixie look Poor academic skills, good verbal and musical abilities-Chromosomal Deletion inv Dup 15 syndrome- Poor muscle tone, mental retardation, seizures, curved spine, and autistic characteristics, Chromosomal Duplication
Alagille Syndrome - Distinctive face, abnormalities of eyes & internal organs, and severe itching. Translocation
Saethre-Chotzen syndrome - Chromosome Inversion
Polyploidy when extra chromosomes are found in an individual, for example trisomy 21 or Downs syndrome
Non disjunction event during Anaphase of Meiosis in which chromosomes fail to separate that can lead to polyploidy
DOWNS SYNDROME =TRISOMY21 (3 copies of a chromosome 21) mental delays, changes in blood vessels and connective tissue
DNA Technology - Genetic Engineering – the use of technology to understand and change gene function
The Human Genome Project Decoded an entire length of DNA in 2003
Genetic Sequencing
– decoded nucleotide sequence for the 23,000+ human genes, allows for the detection and treatment of genetic disease and cancer.
Gene Therapy -good copies of a gene are introduced into those individuals affected by genetic disease, often using viruses
Virus – a non-living vector that infects cells with DNA and uses the cells machinery to reproduce toil the cell lyses or explodes
BIOLOGY CH16 Evolution VOCABULARY
CHARLES DARWIN : The Father of Evolution through Natural Selection, he observed change through “Descent with Modification.” Wrote on the Origin of Species in 1859
SPECIES : Organisms so similar they can interbreed and produce VIABLE (fertile) offspring
NATURAL SELECTION: greater reproductive success displayed by individuals with ADAPTIVE traits
VARIATION: differences within a population
ADAPTATIONS: traits that are selected for because they help an organism survive and reproduce
EVOLUTION: Changes in species over time- Occurs when genes in a population change/shift to enhance survival and reproduction
JEAN BAPTISTE LAMARCK: Observed organisms change/evolve by passing down AQUIRED TRAITS
LYELL AND HUTTON: Geologists who proposed GRADUALISM as the source of change
CUVIER: Geologist who proposed CATASTROPHISM as the source of change
EVIDENCE FOR EVOLUTION: Comes from Fossils, Comparative Anatomy, Embryology, Biochemistry, Biogeography, and others!
FOSSIL EVIDENCE: Records of past organisms show common ancestry
COMPARATIVE ANATOMY: the study of similarities in organisms of common ancestry
Vestigial Structures- appendix, whale pelvis Analogous Structures – bee wing v. bird wing
Homologous structure - your hands-whale flipper
EMBRYOLOGY: The study of how embryos develop to show common ancestry- you had gills and a tail!
BIOCHEMISTRY: The similarity in gene sequence
BIOGEOGRAPHY: The study of how organisms are distibuted today and in the fossil record
3 TYPES OF ADAPTATIONS
STRUCTURAL ADAPTATIONS: Physical feature like a wing to fly or fins to swim
PHYSIOLOGICAL ADAPTATION: Body functions like the production of venom or the ability to tolerate heat
BEHAVIORAL ADAPTATION: When organisms work together like a wolf pack, or school of fish
3 TYPES OF NATURAL SELECTION
DIRECTIONAL SELECTION: When a direction in phenotypes is favored- bigger brains
STABILIZING SELECTION: When 1 average phenotype is selected for – 7 lb babys
DISRUPTIVE SELECTION: When 2 extreme phenotypes are selected for simultaneously- different colored butterflies
SPECIATION: The formation of new species through evolution
Chapter 17 Population Genetics and Speciation VOCABULARY
MICROEVOLUTION: the study of changes in allele frequency of a population
POPULATION: A group of organism of the same species that are breeding
PHENOTYPIC VARIATION: the allele frequencies within a population
NORMAL DISTRIBUTION: the bell curve
ALLELE FREQUENCY: the ratio of specific alleles in a population
GENETIC VARIATION: the different forms of alleles and traits in a population
HARDY WEINBERG EQUILIBRIUM: IS an IDEAL condition (IMPOSSIBLE) in which no evolution or allele frequency change occurs
(EQUILIBRIUM)- when all of the following DO NOT HAPPEN
GENE FLOW: the gain or loss of new alleles through immigration or emmigration
MUTATION: change in DNA sequence
NONRANDOM MATING: reproductive selection, females choose mates selectively
GENETIC DRIFT: small populations can have major changes in allele frequency
NATURAL SELECTION: when the environment favors a particular allele over another
FOUNDER EFFECT: the initial alleles/individuals in a population have great effect on allele frequency
POPULATION SIZE: the larger the population the smaller the impact of individual changes in allele frequency
SPECIATION: the development of new species through directional, disruptive or stabilizing selection
REPRODUCTIVE ISOLATION: a barrier to reproduction amoung individuals that leads to new species
Geography: physical barriers like rivers, canyons, oceans –squirrels in grand canyon
Ecological Niche: organisms are performing different roles in an ecosystem – Darwin Finches
Mating Behavior and Timing: migrations, mating dances in birds
Polyploidy: changes in chromosome number
Hybridization : pairings between similar species- liger