Zinc sulfide quantum dots and polyaniline nanocomposite
advertisement

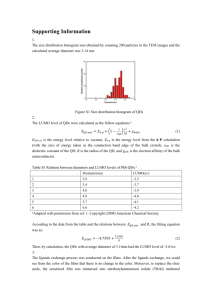
Electronic Supplementary Material Electrochemical clenbuterol immunosensor based on a gold electrode modified with zinc sulfide quantum dots and polyaniline Zhihong Zhang 1,2*, Fenghe Duan1, Linghao He 1, Donglai Peng 1, Fufeng Yan 1, Minghua Wang 2, Wei Zong 3, Chunxiao Jia 1 1 State Laboratory of Surface and Interface Science of Henan Province,Zhengzhou University of Light Industry, No. 166, Science Avenue, Zhengzhou 450001, P. R. China; 2 Henan Collaborative Innovation Center of Environmental Pollution Control and Ecological Restoration, Zhengzhou University of Light Industry, No. 166, Science Avenue, Zhengzhou 450001, P. R. China; 3 Henan Collaborative Innovation Center of Food Production and Safety, Zhengzhou University of Light Industry, No. 166, Science Avenue, Zhengzhou 450001, P. R. China *Corresponding author, Tel.: +0086-37186609676, Fax: +0086-37186609676, ∗ E-mail address: mainzhh@163.com Optical absorbance spectroscopy analysis The UV–visible spectra of ZnS QDs and ZnSQD@PANI nanocomposite are shown in Fig. S1. The absorption peak at 302 nm corresponds to ZnS QDs [1]. In ZnSQD@PANI, the adsorption peaks at 361, 444, and 876 nm correspond to *, polaron band to * band, and band to localized polaron band transitions of PANI, respectively [2]. However, the peak attributed to ZnS QDs is not prominent in the fabricated composite. 1 429 Intensity /a.u. 10.0k ZnS QDs 8.0k 6.0k 417 4.0k ZnSQD@PANI 2.0k 0.0 350 400 450 500 550 600 650 Wavelength / nm Fig. S1 UV-vis absorption spectra of ZnS QDs and ZnSQD@PANI nanocomposite Photoluminescence analysis Photoluminescence spectra of the composite films at room temperature (300 K) and excitation wavelength of 320 nm are shown in Fig. S2. The emission peak at 429 nm is associated with stoichiometric vacancies or defect-related emission of ZnS QDs [2]. The peak exhibits blue shift to 417 nm, which is probably due to changes in particle size or phase of the ZnS nanocrystals [3]. ZnSQD@PANI Intensity 1.5 361 1.0 444 876 0.5 302 ZnS QDs 0.0 200 300 400 500 600 700 800 900 Wavelength/nm Fig. S2 Fluorescence emission spectra of ZnS QDs and ZnSQD@PANI nanocomposite. 2 X-ray photoelectron spectroscopy survey scan of ZnSQD@PANI nanocomposite Intensity /a.u. Zn 2p O 1s C 1s N 1s 1200 1000 800 600 400 S 2p 200 0 Binding Energy / eV Fig. S3 X-ray photoelectron spectroscopy of ZnSQD@PANI composite. Surface morphology of PANI (a) (b) Fig. S4 Scanning electron micrographs of PANI. 3 Cyclic voltammetry diagrams of developed biosensors (a) 150 gold electrode ZnSQD@PANI IgG CHanti CH Current / A 100 50 (b) gold electrode PANI IgG CLBanti 100 Current / A 150 0 -50 -100 50 CLB 0 -50 -100 -150 -0.2 0.0 0.2 0.4 0.6 0.8 -150 -0.2 Potential / V 0.0 0.2 0.4 0.6 0.8 Potential / V (c) gold electrode ZnS QDs IgG CLBanti 100 Current / A 50 CLB 0 -50 -100 -0.2 0.0 0.2 0.4 0.6 0.8 Potential / V Fig. S5 Cyclic voltammetry curves of the developed biosensor based on (a) ZnSQD@PANI nanocomposite, (b) PANI, and (c) ZnS QDs for detecting 1 ng·mL−1 CH. Repeatability of the electrochemical biosensor Table S1 Repeatability of the developed electrochemical biosensor. Times Rct(CH) △ (0.5 ng·mL-1) (kΩ) Rct RSD (n=3) (%) 1 4.19 2.09 0 2 4.08 1.95 0.1 3 4.00 2.04 0.23 4 4.95 1.90 0 5 4.05 1.982 0.146 4 Real samples Table S2 Determination of clenbuterol hydrochloride concentrations spiked in pig urine using the fabricated biosensor. Supplemented Detected amount amount (nmol) (nmol) 1 0.01 2 Recovery RSD (n=3) (%) (%) 0.0097 97 0.27 0.1 0.0964 96.39 0.13 3 0.5 0.510 102 0.15 4 1 0.978 97.85 0.09 5 2 2.06 103 0.13 6 10 11.49 100.15 0.12 Samples References 1 Labiadh H, Chaabane T B, Balan L, Becheik N, Corbel S, Medjahdi G and Schneider R (2014) Preparation of Cu-doped ZnS QDs/TiO2 nanocomposites with high photocatalytic activity. Applied Appl. Catalysis Catal. B: Environmental Environ. 144: 29-35. 2 Shit A, Chatterjee S and Nandi A K (2014) Dye-sensitized solar cell from polyaniline–ZnS nanotubes and its characterization through impedance spectroscopy. Physical Phys. Chemistry Chem. Chemical Chem. Physics Phys. 16: 20079-20088. 3 Dey S K and Sarkar D (2014) Effect of Zn source concentration on structural, optical and electrical properties of zinc sulphide–-polyaniline (ZnS–-PANI) nanocomposite thin films. Journal of. Materials Mater. ScienceScie.: Materials Mater.in Electronics Electron. 25: 5638-5645. 5