Density Lab Worksheet: Measuring Metal Density
advertisement
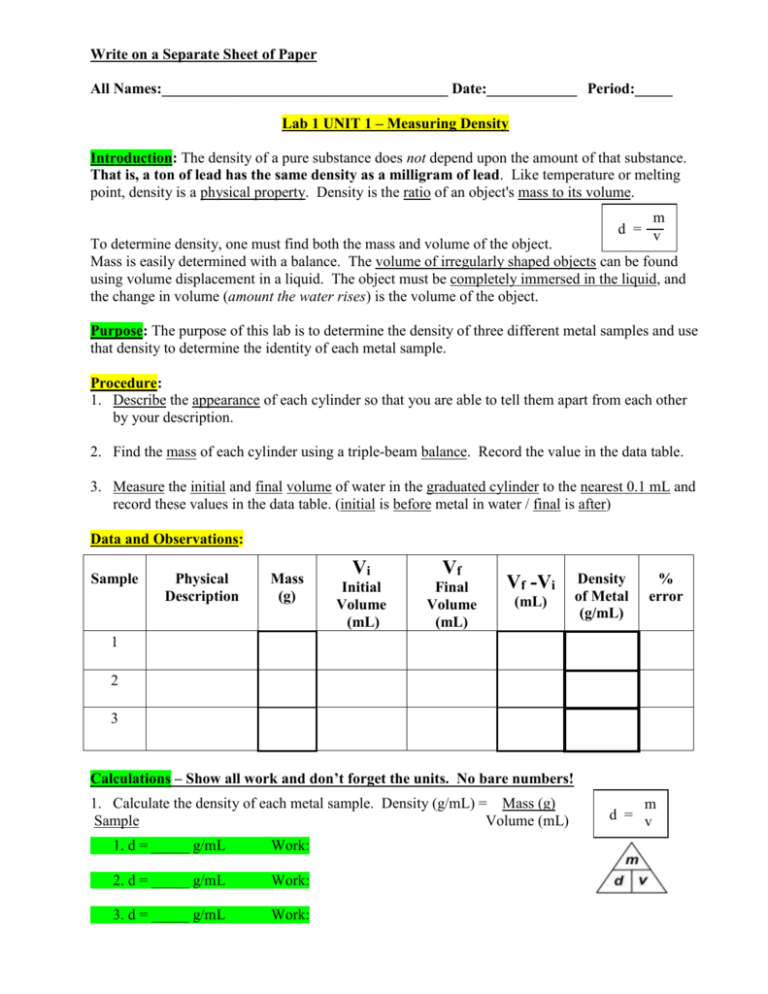
Write on a Separate Sheet of Paper All Names:______________________________________ Date:____________ Period:_____ Lab 1 UNIT 1 – Measuring Density Introduction: The density of a pure substance does not depend upon the amount of that substance. That is, a ton of lead has the same density as a milligram of lead. Like temperature or melting point, density is a physical property. Density is the ratio of an object's mass to its volume. m d = v To determine density, one must find both the mass and volume of the object. Mass is easily determined with a balance. The volume of irregularly shaped objects can be found using volume displacement in a liquid. The object must be completely immersed in the liquid, and the change in volume (amount the water rises) is the volume of the object. Purpose: The purpose of this lab is to determine the density of three different metal samples and use that density to determine the identity of each metal sample. Procedure: 1. Describe the appearance of each cylinder so that you are able to tell them apart from each other by your description. 2. Find the mass of each cylinder using a triple-beam balance. Record the value in the data table. 3. Measure the initial and final volume of water in the graduated cylinder to the nearest 0.1 mL and record these values in the data table. (initial is before metal in water / final is after) Data and Observations: Sample Physical Description Mass (g) Vi Vf Initial Volume (mL) Final Volume (mL) Vf -Vi (mL) Density of Metal (g/mL) % error 1 2 3 Calculations – Show all work and don’t forget the units. No bare numbers! 1. Calculate the density of each metal sample. Density (g/mL) = Mass (g) Sample Volume (mL) 1. d = _____ g/mL Work: 2. d = _____ g/mL Work: 3. d = _____ g/mL Work: m d = v 2. Using the density of the metal samples and the description of each sample, determine the identity of each metal sample by matching it with a metal in the chart this page. Metal Water Aluminum Zinc Copper Silver Lead Density (g/mL) 1.00 2.70 7.13 8.96 10.49 11.36 Metal Water Mercury Gold Iron Tin Titanium Density (g/mL) 1.00 13.55 19.32 7.87 7.28 4.50 Sample 1: ______________ Sample 2: ______________ Sample 3: ______________ 3. Use the actual density (chart above) of each sample to calculate the % error of each of your experimental density values. % error = actual – experimental x 100 % Sample % Error Work Shown actual 1. _____ % = 2. _____ % = 3. _____ % = Questions: 1. For which kind of objects is it easier to use the water displacement method of finding volume in mL instead of measuring the object’s length, width, and height for volume in cm3? ______________________ Explain: ____________________________________________ 2. Why do most rocks sink in water? (hint: it’s not because they’re “heavy”) ___________________________________________________________________________ 3. Give 2 examples of solid substances that float in water. _______________ ______________ Are they less dense or more dense than water? (circle one) 4. Which metal had the lowest % error? Metal name: ________________ % error: _______ % Was this answer accurate or precise? Why?________________________________________ Conclusion: Write a paragraph discussing the lab’s purpose (refer to the first page) and what you observed and learned about calculating density and identifying substances.







