Challenges of Postoperative Management, ICU Care
advertisement

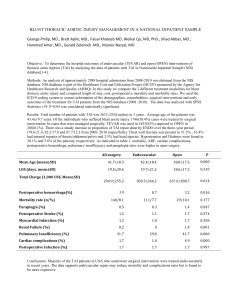
Thoracic Endovascular Aortic Repair (TEVAR) Moderator: E. Andrew Ochroch, MD Challenges of Postoperative Management, ICU Care Jacob T. Gutsche MD Assistant Professor University of Pennsylvania Philadelphia, Pa Objective: Participants will gain knowledge of the peri-operative management challenges with a focus on ICU management after TAA repair. TEVAR is now considered a reasonable alternative to open repair of thoracic aortic aneurysms (TAA). Patients undergoing TEVAR tend to be elderly with multiple comorbidities which may increase the risk of post-operative morbidity and mortality. Complications associated with TEVAR include death, stroke, paralysis due to spinal cord ischemia, respiratory failure, renal insufficiency, and vascular complications. The incidence of major complications associated with TEVAR range from 10-12% in the 30-day postoperative period.(1) A thorough understanding of the technical aspects of the surgery and the associated risks is important to develop care guidelines to limit risk of these complications. In addition, monitoring and rapid diagnosis of complications can facilitate therapy delivery to increase the possibility of recovery. Stroke is a common complication ranging from 2.5-3.6% in modern trials, but has been reported to be as high as 8% in other series.(1) The majority of strokes attributed to TEVAR are a result of atheroemboli from manipulation of the aortic arch with wires and delivery devices.(2) Despite this, an emergent computed tomography(CT) scan should be performed to rule out a hemmorhagic stroke. Furthermore, a large stroke may be diagnosed and may change management because of the higher risk of conversion to hemmorhagic stroke. As such, the treatment of atheroembolic stroke is not amenable to thrombolytic therapy. Management of acute post-operative stroke is mostly supportive includes permissive hypertension, serum glucose control, maintaining normothermia, optimizing the head of bed position.(3) Patients who are able to maintain oxygenation, have a low risk for aspiration, and low suspicion for elevated intracranial pressure should be maintained in supine position.(4) The risk of spinal cord ischemia ranges from 3-11%. Lumbar drain and hemodynamic management to minimize the risk of paralysis will be discussed by Dr. Fedorow. Post-operative renal insufficiency following TEVAR is associated with worse outcome including stroke, myocardial infarction, and paraplegia.(5) Pre-operative renal insufficiency has been shown to increase the risk of converting to renal failure requiring dialysis.(5) The use of contrast during TEVAR deployment may temporarily worsen renal function, practitioners should use low doses of low or iso-osmolar contrast agents when possible. N-acetylcysteine, sodium bicarbonate infusions, or saline hydration been utilized to prevent contrast induced nephropathy (CIN). Several small trials have examined the use of diuretics for CIN prophylaxis, but the results are conflicting.(6,7) Hydration should be judicious since fluid overload may be pooly tolerated in this patient population may be challenging particularly in the case of significant ventricular dysfunction. Vascular complications following TEVAR can include thromboembolism to the mesenteric circulation or extremities . Monitoring in the immediate postoperative period should include extremity pulse checks and examination of the patient for signs or symptoms of ischemia. Mesenteric ischemia may present as vague abdominal pain out of proportion to exam. Furthermore, vascular complications may include access site bleeding leading to retroperitoneal hemorrhage. The cannula used for TEVAR are large and may cause vessel injury, although the absolute risk of this complication is considered rare. Patients with difficult arterial anatomy may require a retroperitoneal approach which is associated with a higher incidence of retroperitoneal hematoma.(8) The extraperitoneal space may conceal a large hematoma, which may delay diagnosis. A high index of suspicion should be maintained in patients with unstable hemodynamics, new abdominal distension and tenderness, or acute anemia requiring transfusion. Other signs which are more subtle include oliguria or increased hepatic enymes. Diagnosis may be confirmed by CT scan, ultrasound, or rarely transesophageal echocardiography. Treatment of retroperitoneal hematoma includes correction of coagulopathy, monitoring for signs of abdominal compartment syndrome, and supportive therapy. Surgical intervention may be necessary in select patients to decompress the hematoma. In summary, the risk of adverse events following TEVAR remains high. An understanding of the procedure, close monitoring, and rapid diagnosis and treatment of complications can improve outcomes. 1. 2. 3. 4. 5. 6. 7. Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE, Jr., Eagle KA, Hermann LK, Isselbacher EM, Kazerooni EA, Kouchoukos NT, Lytle BW, Milewicz DM, Reich DL, Sen S, Shinn JA, Svensson LG, Williams DM, American College of Cardiology Foundation/American Heart Association Task Force on Practice G, American Association for Thoracic S, American College of R, American Stroke A, Society of Cardiovascular A, Society for Cardiovascular A, Interventions, Society of Interventional R, Society of Thoracic S, Society for Vascular M. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation 2010;121:e266-369. Gutsche JT, Cheung AT, McGarvey ML, Moser WG, Szeto W, Carpenter JP, Fairman RM, Pochettino A, Bavaria JE. Risk factors for perioperative stroke after thoracic endovascular aortic repair. The Annals of thoracic surgery 2007;84:1195-200; discussion 200. Jauch EC, Saver JL, Adams HP, Jr., Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Jr., Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H, American Heart Association Stroke C, Council on Cardiovascular N, Council on Peripheral Vascular D, Council on Clinical C. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke; a journal of cerebral circulation 2013;44:870947. Wojner-Alexander AW, Garami Z, Chernyshev OY, Alexandrov AV. Heads down: flat positioning improves blood flow velocity in acute ischemic stroke. Neurology 2005;64:1354-7. Wang GJ, Fairman RM, Jackson BM, Szeto WY, Pochettino A, Woo EY. The outcome of thoracic endovascular aortic repair (TEVAR) in patients with renal insufficiency. Journal of vascular surgery 2009;49:42-6. Solomon R, Werner C, Mann D, D'Elia J, Silva P. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. The New England journal of medicine 1994;331:1416-20. Marenzi G, Ferrari C, Marana I, Assanelli E, De Metrio M, Teruzzi G, Veglia F, Fabbiocchi F, Montorsi P, Bartorelli AL. Prevention of contrast nephropathy by furosemide with matched hydration: the MYTHOS (Induced Diuresis With Matched Hydration Compared to Standard Hydration for Contrast Induced Nephropathy Prevention) trial. JACC Cardiovascular interventions 2012;5:90-7. 8. Etezadi V, Katzen BT, Benenati JF, Alehashemi S, Tsoukas AI, Puente OA. Retroperitoneal versus direct femoral artery approach for thoracic endovascular aortic repair access: a case-control study. Annals of vascular surgery 2011;25:340-4.