Falsely lowered glycosylated hemoglobin levels in a patient with
advertisement

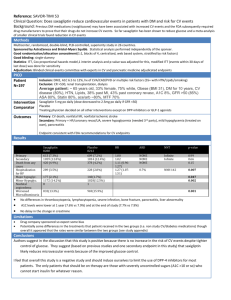
Falsely lowered glycated hemoglobin levels in a patient with hemochromatosis and type 2 diabetes mellitus Beverly Mielke Kocarnik MD, MPH1*, Dawn DeWitt MD, MSc2, Irl B. Hirsch MD3 1Department of Internal Medicine, Residency Training Program, University of Washington School of Medicine, Box 356421, 1959 NE Pacific St, Seattle, WA, 98195, 2Rural Health Academic Centre, Melbourne Medical School, The University of Melbourne, 49 Graham Street, Shepparton VIC 3632, Australia, 3Division of Metabolism, Endocrinology, & Metabolism, University of Washington School of Medicine, 4225 Roosevelt Ave NE, Suite 101, Seattle, WA, 98105, Running title: Glycated hemoglobin and hemochromatosis Email: Beverly Mielke Kocarnik bmielke@u.washington.edu; Dawn DeWitt ddewitt@unimelb.edu.au; Irl B. Hirsch ihirsch@u.washington.edu *Corresponding author: Beverly Mielke Kocarnik MD, MPH Department of Internal Medicine Residency Training Program University of Washington School of Medicine Box 356421 1959 NE Pacific Street Seattle, WA 98195 Email: bmielke@uw.edu Phone: 971-404-7060 Fax: 206-685-8652 Abstract word count: 187 Article text word count: 1166 Number of tables and figures: 2 Keywords: hemochromatosis, glycated hemoglobin, diabetes mellitus 1 Abstract Diabetes occurs in approximately 50% of patients with hereditary hemochromatosis. Glycated hemoglobin (A1C) measurements are inaccurate in these patients since they undergo intermittent phlebotomy with secondarily induced red cell production. Most primary care physicians will care for at least one patient with these concomitant diseases, and this case demonstrates the difficulty of interpreting A1C values in this population. A 59 year old Caucasian male with a history of type 2 diabetes and hereditary hemochromatosis was referred for evaluation of discordant fasting blood glucose and A1C levels. His A1C level decreased from 7.4% to 6.1% during a 2 month period while fasting home blood glucose levels were stable at 712 mmol/liter. Minimal changes had been made to his medications, and he had made no significant lifestyle changes to account for this decrease. He received phlebotomy twice during this interval. Since most primary care physicians will care for several patients with hereditary hemochromatosis and diabetes mellitus, we hope to increase awareness about the challenges of A1C interpretation in this patient population. A1C is unreliable in patients with hemachromatosis and should not be used for diagnosis, monitoring, and treatment decisions. 2 Introduction Hereditary hemochromatosis is an autosomal recessive disorder that affects between 1 in 200 and 1 in 500 Caucasians.1 Half of these patients will have either type 1 or type 2 diabetes.2 Therefore, an average primary care panel with 1,500 patients will include approximately 4 patients with both hereditary hemochromatosis and diabetes. In this report, we discuss a patient with concurrent hereditary hemochromatosis and diabetes whose glycated hemoglobin levels (A1C) appeared to be inaccurate relative to his home capillary blood glucose readings and fasting blood glucose values. Since the vast majority of primary care physicians will have several patients with these concurrent diseases, it is important to understand the optimal management and diabetes monitoring for such patients, especially in light of the American Diabetes Association recommendation that A1C can be used for diagnosis of diabetes.3 Case Presentation A 59 year old Caucasian male with hemochromatosis and diabetes was referred for discordant fasting blood glucose and glycated hemoglobin levels (A1C). He was diagnosed with diabetes fifteen years previously and had been treated with oral agents for several years. His A1C remained below 7% until 6 months prior to consultation. At the time of referral, he was taking metformin 1500 mg daily and glimepiride 2 mg every morning. Additional medications 3 were hydrochlorothiazide/irbesartan 12.5 mg/300 mg daily, aspirin 100 mg daily, and sildenafil 50 mg as needed. The patient’s physical examination showed truncal obesity with a BMI of 38, but was otherwise unremarkable without hyperpigmentation. When the patient’s A1C rose above 7%, he began recording his home blood glucose readings. Morning fasting glucose values ranged from 7.0 mmol/L to 12.0 mmol/L, with an average of 8.7 mmol/L over 105 morning readings during the prior 6 month. Three fasting plasma glucose levels during this time period were significantly elevated (9.4 to 10.6 mmol/L). However, his A1C levels over that time period were 6.8%, 7.0%, 7.4%, and 6.1% in chronological order. Table 1 shows a time-correlation of patient results and treatment. The most pronounced change in his A1C levels was the decrease from 7.4% to 6.1%. Despite this decrease in A1C, there had been no change in his home blood glucose reading measurements. The readings corresponding to the 3-month period before the A1C value of 7.4% showed a mean glucose of 8.74 mmol/L with a standard deviation of 0.98 mmol/L, and readings corresponding to the 3-month period before the A1C value of 6.1% had a mean of 8.77 mmol/L (SD 0.88). His medication regimen was changed from glimepiride 2 mg every morning to gliclazide 60 mg daily 26 days prior to the lower A1C value (6.1%). This change was effectively equivalent to a lower dose of sulfonylurea.4 The only other recent change in his medication regimen was the initiation of amlodipine 5 mg daily for hypertension. The patient’s hemochromatosis was diagnosed approximately 11 years prior on laboratory testing for the evaluation of arthropathy, which had since resolved. Liver function tests were normal on three occasions in the past year. His diabetes may be due to obesity or 4 hemochromatosis. He typically undergoes phlebotomy every 3 to 4 months, although had closely spaced phlebotomy in December 2010 and January 2011 and has not required phlebotomy since then. Other laboratory studies revealed a C-peptide in the low normal range at 0.6 nmol/L (normal 0.3-2.3 nmol/L). Fructosamine was 349 umol/L (normal 205-285 umol/L) when his A1C was 7.4%. Concurrent thyroid stimulating hormone and fasting lipids were within normal limits except for an elevated triglyceride level of 1.9 mmol/L (normal <1.5 mmol/L). Discussion This patient’s A1C does not appear to accurately reflect the average blood glucose levels for this patient as evidenced by the drop in his A1C from 7.4% to 6.1% over a 2 month time period. The only changes during this period were a switch in his sulfonylurea regimen and phlebotomy 26 days prior to the lower A1C. One milligram of glimepiride is equivalent to 80 mg of gliclazide, so his dosage of sulfonylurea was relatively lower with the change and does not explain the decrease in his A1C4. Therefore, his phlebotomy with subsequent red cell production is the most likely explanation for the “improved A1C”, given the nearly identical home glucose monitoring values with different A1C readings. See table 2 for the correlation of A1C and average blood glucose. The discrepancy between his A1C and fructosamine in the context of his home blood glucose tests is also consistent with a falsely low A1C. 5 The most likely explanation for the lower values of A1C after phlebotomy is the subsequent induction of increased red blood cell production. These new cells have less time available for glycation, so the A1C appears falsely low.5,6 Conditions resulting in a shorter lifetime of an average red blood cell have been shown to decrease A1C, although hemochromatosis is often omitted.7 Additionally, red blood cell production in patients with hemochromatosis, as measured by reticulocyte count prior to phlebotomy, is highly inconsistent and probably contributes to false A1c readings.8 We hope this case will alert health care professionals to the inaccuracies of A1C among patients with hemochromatosis who are undergoing regular phlebotomy. Iron-deficiency anemia with hypoproliferation of red cells results in a higher than expected A1C but hemochromatosis, especially with phlebotomy (and increased reticulocytes) should cause a falsely low A1c in most patients. Increasing frequency of phlebotomy should theoretically lead to increasingly inaccurate A1C results. Practitioners should keep this in mind when evaluating and optimizing glycemic control in patients with hemochromatosis. Importantly, as physicians move to the new recommendations for using A1C for diagnosis, we recommend that standard glucose diagnostic measures (fasting, random or glucose tolerance test) be used in patients with hemochromatosis, who would be under-diagnosed using A1C. Finally, this patient’s A1C appears to be approximately 0.5% lower (at least) than expected from home blood glucose monitoring. If a decrease of A1C by 1% lowers complications by 30% then this patient’s A1C treatment goal should be 6-6.5% rather than the standard <7% when receiving phlebotomy.9 This goal is validated by the patient having virtually no hypoglycemia with an A1C at 6.1%. 6 Conclusion Inappropriately low A1C values in patients with hereditary hemochromatosis and type 2 diabetes mellitus should be expected when patients are undergoing regular phlebotomy since the younger population of red blood cells has less time to be glycated. In addition, hemochromatosis itself may result in false A1c levels, but further research is required. Since most primary care physicians will care for several patients with concurrent diabetes mellitus and hemochromatosis, awareness of the inaccuracies of measurement among this patient population is important. A1c should not be used for diagnosis, monitoring, or treatment. Home blood glucose monitoring should be the major method used to assess glycemia. In the future, other tests such as fructosamine, glycated albumin and 1,5-anhydroglucitol may be useful as other measures of glucose control, but further validation is required. We hope this case report leads to greater awareness of the inaccuracy of A1c testing among patients with hemochromatosis and promotes further research about the optimal care of this population. Acknowledgments and Conflicts of Interest We would like to thank the patient discussed in this case report for granting permission to present his case. Beverly Mielke Kocarnik and Dawn DeWitt report that they have no conflicts of interest to disclose. Irl Hirsch has received research support from Novo Nordisk, Halozyme, SanofiDiabertes. He is a consultant for Johnson & Johnson, Roche, Abbott Diabetes Care, and Cellnovo. 7 References 1. Hatunic M, Finucane FM, Brennan AM, Norris S, Pacini G, Nolan JJ: Effect of iron overload on glucose metabolism in patients with hereditary hemochromatosis. Metabolism Clinical and Experimental 2010, 59:380-384. 2. Capell P: Case study: Hemochromatosis in type 2 diabetes. Clinical Diabetes 2004, 22:101102. 3. American Diabetes Association. Standards of medical care in diabetes- 2011. Diabetes Care 2011,34:S11-S61. 4. Robkamp R, Wernicke-Panten K, Drager E: Clinical profile of the novel sulfonylurea glimepiride. Diabetes Res Clin Pract 1996, 31(Suppl):s33-s42. 5. Eschwege E, Saddi R, Wacjman H, Levy R, Thibult N, Duchateau A: Haemoglobin A1c in patients on venesection therapy for haemochromatosis (author’s transl). Diabete Metab 1982, 8:137-40. 6. Saddi R, Wajcman H, Eschwege E, Levy R, Duchateau A: Glycated haemoglobin in patient on venesection therapy for haemochromatosis. Lancet 1980, 316:141-2. 7. Gallagher EJ, Le Roith D, Bloomgarden Z: Review of hemoglobin A1c in the management of diabetes. Journal of Diabetes 2009;1:9-17. 8. Sargent T, Saito H, and Winchell HS: Iron absorption in hemochromatosis before and after phlebotomy therapy. Journal of Nuclear Medicine 1972;12:660-7. 9. UK Prospective Diabetes Study (UKPDS) group. Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53. 10. Nathan DM, Kuenen J, Borg R, Sheng H, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care, 2008;31.8:1473(6). 11. American Diabetes Association. DiabetesPro Glucose Calculator. Available at: http://professional.diabetes.org/GlucoseCalculator.aspx. Accessed December 13, 2011. 8 Tables Table 1. Comparative measures of the patient’s diabetes status. Clinic visits A1C 46% Glucose readings HBG or FBG1 (mmol/L) Fructosamine (Normal = 205285 umol/L) Comments July 2010 (referral information) 7.0% FBG 8-10 Hb 158 = Hct 47; ferritin = 114 Phlebotomy Aug 10, 2010 Nov 25 2010 7.4% HBG Average 8.74 349 (Dec 3 + 0.98 (SD) 2010) Sulfonylurea switched (dose equivalent actually lower); cpeptide 0.6 nmol/L (0.3-3.20); BMI 38 Phlebotomy Dec 15, 2010 Jan 20 2011 Jan 31 2011 Insulin glargine initiated at 10 units (bedtime) with selftitration to fasting goal < 6 mmol/L FBG 6.1% HBG Average 8.77± 0.88 (SD) Phlebotomy Jan 5, 2011 April 15 2011 6.4% July 17 2011 1HBG 6.1% Phlebotomy 3 months prior; Hb 156 = Hct 45; BMI 36. Insulin glargine dose 22 units nightly. HBG Average 7.4 = 30 day meter average (25% fasting) 287 Ferritin 100; iron 27.7; Hb 155=Hct 45; MCV 90; BMI 35.6 = home blood glucose; FBG = fasting (capillary) blood glucose 9 Table 2. Correlation of A1C with glucose.10,11 Mean A1C in adults without diabetes is ~5% (average blood glucose = 4 mmol/L). A1C Average blood glucose* 5% 5.4 mmol/L 97 mg/dL 6% 7.0 mmol/L 126 mg/dL 7% 8.6 mmol/L 154 mg/dL 8% 10.2 mmol/L 183 mg/dL 9% 11.8 mmol/L 212 mg/dL 10% 13.4 mmol/L 240 mg/dL *Estimated Average blood glucose: mmol/L ABG = 2 x A1C – 6 mmol/L 10