The Common Ion Effect
advertisement

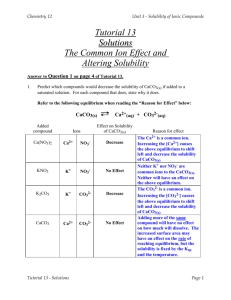
The Common Ion Effect The common ion effect is another example of Le Châtelier's Principle in action. The common ion effect tells us that the solubility of an ionic compound is decreased by the addition to the solution of another ionic compound that contains one of the ions involved in the solution equilibrium. An example should help clarify what happens: If we have a barium sulfate solution, the solid salt is in equilibrium with its ions: BaSO4 (s) Ba2+(aq) + SO42-(aq) If we then add solid barium chloride to this solution, which dissolves to produce Ba2+ and Cl- ions, we are increasing the concentration of Ba2+ ions in our solution. (The new Cl- ions will remain in solution as spectator ions). Ba2+ is the ion common to both solutions. Le Châtelier's Principle tells us that if the concentration of one of the reaction participants is increased, then equilibrium will shift to use up the additional substance. So adding more Ba2+ will force the equilibrium to shift to the left (the reverse direction) in order to use up the added Ba2+ ions, producing more solid BaSO4. The concentration of SO42- will decrease, indicating that solubility has decreased. PRACTICE QUESTION: Describe how the addition of hydrofluoric acid to a saturated solution of potassium fluoride would affect the solution. (HINT: Write the balanced dissociation equation for potassium fluoride first and then think about what ions are being added from the solution of hydrofluoric acid). Factors Affecting Solubility Because a saturated solution is an equilibrium system, the same factors that affect a system at equilibrium will affect our saturated system. The two key factors to consider are: 1. the effect of temperature and 2. the effect of pressure on the solubility of gases. The Effect of Temperature on Solubility If you want to get more salt to dissolve in our glass of water, what would you do? If you heated the water you would be able to dissolve more salt. Generally, increasing the temperature will increase solubility of solids and liquids. But increasing temperature will lower the solubility of gases (the gas will escape from solution, going back to the gas phase). We can explain this using Le Châtelier's Principle. The dissolving process of molecular substances in the solid and liquid form is an endothermic process: solid or liquid + heat aqueous Adding heat will favor the endothermic direction; in this case favoring the product, or aqueous, side of the reaction. The dissolving process of gases, however, is exothermic: gas aqueous + heat Adding heat to this system will again favor the endothermic reaction, which in this case is the reverse reaction. The substance will come out of the dissolved, aqueous state and form back into a gas. The Effect of Pressure on the Solubility of Gases The solubility of gases increases when the pressure above the gas is increased. In other words, more gas will dissolve when pressure is increased. This can again be explained by Le Châtelier's Principle. As our example we will examine a can of coke, or other carbonated beverage. Carbonated beverages contain carbon dioxide which is dissolved under pressure. Our equilibrium system is: CO2 (g) CO2 (aq) On the reactant side of the equation is one mole of gas; on the product side are zero moles of gas. Increasing pressure will favor the side with the fewest moles of gas - in this case the dissolved carbon dioxide. When you open a can of pop, you increase the volume (the entire room versus the inside of the can), thereby decreasing the pressure. According to Le Châtelier's Principle, this will favor the side with the most moles of gas. And that's what happens - when you open a can of pop it begins to fizz - this is the CO2 coming out of solution. The "fizz" are the gas bubbles forming and popping