hOLIDAY HOME WORK CLASS-XII - Kendriya Vidyalaya Karauli
advertisement

HOLIDAY HOME WORK (SUMMER 2015-16)
CLASS –XII
CHEMISTRY
1. Silver crystallises in fcc lattice. If edge length of the cell is 4.07 × 10–8cm and density is 10.5 g cm–3,
calculate the atomic mass of silver.
2. A cubic solid is made of two elements P and Q. Atoms of Q are at the corners of the cube and P at the
body-centre. What is the formula of the compound? What are the coordination numbers of P and Q?
3 Niobium crystallises in body-centred cubic structure. If density is 8.55g cm–3, calculate atomic radius of
niobium using its atomic mass 93 u.
4 If the radius of the octahedral void is r and radius of the atoms in closepackingis R, derive relation between
r and R.
5 Copper crystallises into a fcc lattice with edge length 3.61 × 10–8 cm.Show that the calculated density is in
agreement with its measuredvalue of 8.92 g cm–3.
6 Analysis shows that nickel oxide has the formula Ni0.98O1.00. What fractionsof nickel exist as Ni2+ and
Ni3+ ions?
7 What is a semiconductor? Describe the two main types of semiconductorsand contrast their conduction
mechanism.
8 Non-stoichiometric cuprous oxide, Cu2O can be prepared in laboratory.In this oxide, copper to oxygen ratio
is slightly less than 2:1. Can youaccount for the fact that this substance is a p-type semiconductor?
9 Ferric oxide crystallises in a hexagonal close-packed array of oxide ionswith two out of every three
octahedral holes occupied by ferric ions.Derive the formula of the ferric oxide.
10. The time required for 10% completion of a first order reaction at 298K is equal to that required for its 25%
completion at 308K. If the value of A is 4 × 1010s–1. Calculate k at 318K and Ea.
11 Why do gases always tend to be less soluble in liquids as the temperatureis raised?
12 State Henry’s law and mention some important applications?
13 The partial pressure of ethane over a solution containing 6.56 × 10–3 g ofethane is 1 bar. If the solution
contains 5.00 × 10–2 g of ethane, then whatshall be the partial pressure of the gas?
14 What is meant by positive and negative deviations from Raoult's law and how isthe sign of ΔmixH related
to positive and negative deviations from Raoult's law?
15 An aqueous solution of 2% non-volatile solute exerts a pressure of 1.004 bar at the normal boiling point of
the solvent. What is the molar mass of the solute?
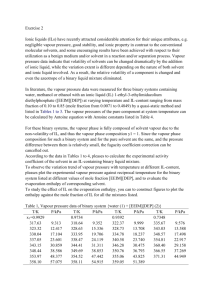
16 Heptane and octane form an ideal solution. At 373 K, the vapour pressures ofthe two liquid components
are 105.2 kPa and 46.8 kPa respectively. What willbe the vapour pressure of a mixture of 26.0 g of heptane
and 35 g of octane?
17 The vapour pressure of water is 12.3 kPa at 300 K. Calculate vapour pressureof 1 molal solution of a nonvolatile solute in it.
18 Calculate the mass of a non-volatile solute (molar mass 40 g mol–1) whichshould be dissolved in 114 g
octane to reduce its vapour pressure to 80%.
19 A solution containing 30 g of non-volatile solute exactly in 90 g of water has avapour pressure of 2.8 kPa at
298 K. Further, 18 g of water is then added tothe solution and the new vapour pressure becomes 2.9 kPa at
298 K. Calculate:
(i) molar mass of the solute
(ii) vapour pressure of water at 298 K.
20 A 5% solution (by mass) of cane sugar in water has freezing point of 271K.Calculate the freezing point of
5% glucose in water if freezing point of purewater is 273.15 K.
21 Conductivity of 0.00241 M acetic acid is 7.896 × 10–5 S cm–1. Calculate itsmolar conductivity. If 0m L for
acetic acid is 390.5 S cm2 mol–1, what is itsdissociation constant?
22 How much charge is required for the following reductions:
(i) 1 mol of Al3+ to Al?
(ii) 1 mol of Cu2+ to Cu?
(iii) 1 mol of MnO4– to Mn2+?
23 How much electricity in terms of Faraday is required to produce
(i) 20.0 g of Ca from molten CaCl2?\
(ii) 40.0 g of Al from molten Al2O3?
24 How much electricity is required in coulomb for the oxidation of
(i) 1 mol of H2O to O2?
(ii) 1 mol of FeO to Fe2O3?
25 A solution of Ni(NO3)2 is electrolysed between platinum electrodes using acurrent of 5 amperes for 20
minutes. What mass of Ni is deposited at thecathode?
26. The rate constant for the first order decomposition of H2O2 is given by the following equation: log k =
14.34 – 1.25 × 104K/T Calculate Ea for this reaction and at what temperature will its half-period be 256
minutes?
27. The rate constant for the decomposition of hydrocarbons is 2.418 × 10–5s–1 at 546 K. If the energy of
activation is 179.9 kJ/mol, what will be the valueof pre-exponential factor.
28. Consider a certain reaction A → Products with k = 2.0 × 10–2s–1. Calculate the concentration of A
remaining after 100 s if the initial concentration of A is 1.0 mol L–1.
29. The decomposition of hydrocarbon follows the equation k = (4.5 × 1011s–1) e-28000K/T Calculate Ea.
Summer vacation home work for class -12
List of investigatory projects assigned to each student:1. Plant hormones and their effect on plant growth- poonam tiwari
2. Evola virus:
Megha sharma
3. HIV/AIDSAnkita singh
4. CancerAnju kushwah
5. TuberculosisShivani
6. DNA fingerprintingAnjali
7. Recombinant DNA technologyAbhishek
8. Sewage and its treatmentTarun
9. Population interactionSagar
10.Mineral nutrients and their importance to plants-Hemendra
11.Swine fluSunny
12.Human brainSudhansu
13.Environmental pollutantsMayank shekhar jaga
Que 1- Write about the conservation strategies to save biodiversity.
Que-2 What are population interactions? Write with examples.
Que.3 How micro organisms are useful to us? Write with examples.
Que.4 Write a note on assisted reproductive techniques.
Que.5 What are STD’s ? Write with examples.
Summer Holiday Homework
Session-2015-16
Class-XII
Sub- English Core
Given By- Rakesh Kumar Gill (PGT Eng)
Q.1. Read the novel “The Invisible Man” intensively covering all the chapters from 01 to 28.
Q2. Solve previous year A.I.S.S.E English core paper (any one set) thoroughly in your notebook excluding the
literature and novel section.
Q.3. Make a file which consist of the following exercises :-(1) LETTERS
(Total- 5)
Job application+ complain+ Order placement +cancellation (One of each type)
(2) ARTICLES
(Total- 5)
Based on Current problems+ Social problems+ any other relevant issues
(3) ADVERTISEMENT
(Total- 5)
Opening new office +To-Let+ Matrimonial+ For Salel+ To Purchase
(4) SPEECH
(Total- 5)
Any relevant issue from the social scenario which are of grave concern for human life and development may
be taken.
KENDRIYA VIDYALAYA KARAULI
HOLIDAYS HOMEWORK (SUMMER VACATION)
CLASS-XII SUB.-MATHS
Q.1. Let * be binary operation defined on R by a * b = 1 + ab, a, b ∈ R. Then the operation * is (i) commutative
but not associative (ii) associative but not commutative (iii) neither commutative nor associative (iv) both
commutative and associative
Q.2. Functions f , g : R → R are defined, respectively, by f (x) = x2 + 3x + 1, g (x) = 2x – 3, find (i) f o g (ii) g o f
(iii) f o f (iv) g o g
Q.3. Let A = {1, 2, 3, ... 9} and R be the relation in A ×A defined by (a, b) R (c, d) if a + d = b + c for (a, b), (c, d)
in A ×A. Prove that R is an equivalence relation and also obtain the equivalent class [(2, 5)].
Q.4. Each of the following defines a relation on N: (i) x is greater than y, x, y€ N (ii) x + y = 10, x, y€ N (iii) x y is
square of an integer x, y€ N (iv) x + 4y = 10 x, y€ N. Determine which of the above relations are reflexive,
symmetric and transitive.
Q.6. Let R be relation defined on the set of natural number N as follows: R = {(x, y): x N, y N, 2x + y = 41}. Find
the domain and range of the relation R. Also verify whether R is reflexive, symmetric and transitive
𝑥−2
Q.7. Let A = R – {3}, B = R – {1}. Let f : A → B be defined by f (x) = 𝑥−3 , x€A . Then show that f is bijective
Let n be a fixed positive integer. Define a relation R in Z as follows: a, b€ Z, aRb if and only if a – b is divisible
by n . Show that R is an equivalance relation.
Q.8. Let the function f : R → R be defined by f (x) = cosx, ∀ x ∈ R. Show that f is neither one-one nor onto
If the mappings f and g are given by f = {(1, 2), (3, 5), (4, 1)} and g = {(2, 3), (5, 1), (1, 3)}, write f o g.
Q.9.Let R be the set of real numbers and f : R → R be the function defined by f (x) = 4x + 5. Show that f is
invertible and find f –1.
Q.10. Consider a function f :R+[-5, ∞) defined f(x) = 9x2 +6x – 5. Show that f is invertible &
f -1(y) =[√𝑦 + 6 − 1]/3, where R+ = (0,∞).
7𝜋
5𝜋
Q.11. Find the principal value of cos-1(cos 6 ) Ans: 6
Q.12.Prove that tan-1
(√1+𝑥 2 +√1−𝑥 2 )
(√1+𝑥 2 −√1−𝑥 2 )
1
𝜋 1
= 4 +2 cos-1x2
1
Q.13.Show that cos[2tan-17 ] = sin(4tan-13 )
1Mark
4Marks
SOME PROBLEMS ARE FROM NCERT EXAMPLAR BOOK (MATHS) FOR CLASS-XII.
HOLLYDAY HOME WORK (SUMMER)
CLASS-XII (PHYSICS)
1. ELECTRIC CHARGES & FIELD
(A) EXAMPLES
(B) EXERCISE
© NUMERICALS
2. ELECTRIC POTENTIAL & CAPACITANCE
(A) EXAMPLES
(B) EXERCISE
© NUMERICALS
3.THREE PRACTICALS IN PRACTICAL FILE
4.CBSE QUESTION OF LAST THREE YEARS OF UNIT-I