File
advertisement

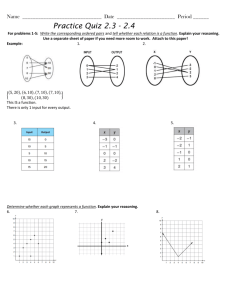
Name _____________________________ Date _______________ Period_____________ ISN Page _______ Unit 2 Assessment Review **I will be able to explain that matter has properties that are determined by the structure and arrangement of its atoms. 1. Classify each of the following statements below into the appropriate category: - Has the most amount of energy - Particles are far apart and move - Attraction between particles is very strong randomly - Has the least amount of energy - Have no definite shape, but take on the - Has more energy than solids and less shape of their container. energy than gases - Particles are close together and vibrate in - Particles are close together and move place. randomly - Attraction between particles is moderate - Have a definite shape and volume - Have no definite shape or volume (can be - Attraction between particles is very weak compressed) Gas Liquid Solid 2. On the right, you’ll see the particle arrangement of a substance. On the line below the picture, identify it as a solid, liquid, or gas. If I add heat to the substance, what will happen? Draw the particle arrangement in the empty box. ______________ 3. Dry ice is used in fire extinguishers. The dry ice is stored in the cylinder in a solid form. When sprayed on a fire, it quickly changes into the gas known as carbon dioxide (CO2). What is this change in state called? __________________________ 4. The diagram shows a change of state in water. What phase change does the X represent? _______________________ 5. When the temperature of a liquid is increased a little, what happens to its particles? A. The particles start vibrating more B. The particles move so close together that the liquid becomes a gas C. The particles stop vibrating D. The particles lose mass Explain your reasoning: ___________________________________________________________________________________________ ___________________________________________________________________________________________ ___________________________________________________________________________________________ ___________________________________________________________________________________________ **I will be able to apply an equation to determine the density of an object based on its mass and volume. For each of the below questions, make sure you show your work and put units on your final answer. 6. The volume of a solution was measured in a graduated cylinder (shown to the right). If the mass of the solution is measured to be 90 grams, what is the density of the solution? 7. The density of aluminum is 2.7 g/mL. If the mass of a piece of aluminum is 244 grams, what is the volume of the aluminum? 8. Why does ice float on top of liquid water? A. Ice has a lower density than water B. Ice has a higher density than water C. Ice is a solid D. Ice is colder than water Explain your reasoning for your answer choice. _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ _______________________________________________________________ #9- Multiple Answers could be correct. Choose the correct answer(s) and justify your reasoning. 9. Why is density considered a useful property for identifying matter? A. Different substances have the same densities B. Density is a property that is unique to each substance C. Density predicts whether objects will change state D. Density varies at different temperature E. Density can determine how pure a substance is F. Density identifies the weight of a substance Explain your reasoning for your answer choice. _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ ________ Explain your reasoning for your answer choice. _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ _______________________________________________ ________Be sure to color and label each of the 11. Below are densities of various liquids. Layer the liquids in the column. 10. Why doesn’t an ice cube float in air? A. Water is less dense than air B. Air is more dense than ice C. An ice cube is more dense than air D. Air has more pressure substances. Substance Sea water Glycerin Cooking oil Water Ethanol Sulfuric acid Gasoline Density (g/cm3) 1.02 1.26 0.92 1.00 0.79 1.83 0.66 **I will be able to interpret the results of an investigation to determine whether a physical or chemical change has occurred. 12. Ms. Schooley was conducting an experiment to determine what type of environment produces rust. She decided to set up three different environments and place an iron nail in each environment. She closely monitored the experiment and wrote down the time that rust began to appear on the nail. Below are her results: Salt water Bottled water Tap water Filtered water Rusts in 36 hours Rusts in 76 hours Rusts in 74 hours Rusts in 85 hours A. Is rusting a physical or chemical change? Why or why not? _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________ B. Identify the independent variable _________________________________________________________________ C. Identify the dependent variable __________________________________________________________________ D. Identify 3 variables that should be controlled in this experiment a. _________________ b. _____________________ c. ___________________ 13. The evil scientist, Ms. Asbill, challenges her students by telling them that they must blow up a balloon using only science! Ms. Asbill cackles into the sky and tells her students that there is only one rule for this challenge: students are not allowed to blow up the balloon themselves. Students are given a random list of supplies to use to design their experiment. Sam, Kendall, and Jarod decide to combine yeast, sugar, and water into an Erlenmeyer flask. They quickly secure the balloon on top of the flask and sure enough- the balloon inflates!! Is the result classified as a physical or chemical change? Explain. ___________________________________________________________________________________________ ___________________________________________________________________________________________ ___________________________________________________________________________________________ ___________________________________________________________________________________________ 14. Mrs. Stamper has gone berserk! When Ameilia, Elijah, and Caitlan arrive to class, they see the frenzied, crazyeyed Mrs. Stamper smash her reading glasses while running to her stack of ungraded papers. She grabs the ungraded papers and shreds them with her mighty fists! Then, she jumps on a chair and breaks the back of the chair off! She even crushed an empty can of coke on her forehead! Ameilia, Elijah, and Caitlan run to their teacher and exclaim, “Mrs. Stamper! Mrs. Stamper! What’s gotten into you? Are you okay?” Mrs. Stamper giggles and growls, “I need chocolate!” Alex suddenly appears in the classroom and throws Milky Ways and Twix at Mrs. Stamper. She consumes the candy immediately, but it’s not enough! The ravenous Mrs. Stamper is now demanding brownies from the CDC. The concerned students run to the CDC and ask at Mrs. Youngquist to make more brownies to appease Mrs. Stamper! Luckily, a fresh batch is out of the oven. The students take the brownies back to Mrs. Stamper. After eating a brownie, Mrs. Stamper returns to her normal self. Identify the physical and chemical changes in the story above. (Hint: At least 3 physical changes and 2 chemical changes) Physical Changes Chemical Changes