Grade 10 Physical Sciences Exam Paper 2014
advertisement

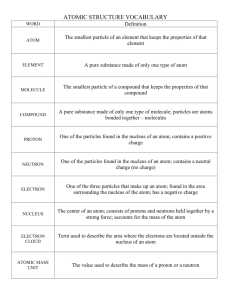
GRADE10 : PHYSICAL SCIENCES PAPER 2 JUNE EXAMINATION 2014 MARKS: 150 DURATION: 2 hours This question paper consists of 11 pages (including this one) Physical Sciences grade 10 P2 2014 June Examinations INSTRUCTIONS AND INFORMATION 1 Answer all questions. 2 Non-programmable calculators may be used. 3 Appropriate mathematical instruments may be used. 4 Number the answers correctly according to the numbering system used in this question paper. 5 Be brief whenever motivations, discussions, et cetera, are required 6 Where needed, data sheets and graph papers will be provided by the school. 7 8 Leave ONE line between two sub-questions, for example between QUESTION 3.1 and QUESTION 3.2. Show the formulae and substitutions in ALL calculations. 9 Round off your final answer to two (2) decimal places, unless otherwise stated. Page 2 of 11 Physical Sciences grade 10 P2 2014 June Examinations QUESTION 1 MULTIPLE CHOICE QUESTIONS Four options are provided as possible answers to the following questions. Each question has only one correct answer. Write the letter ( A – D) of the correct answer next to the question number( 1.1 – 1.10) 1.1 a…………..is a mixture that is uniform where the different components of the mixture (2) cannot be seen. A Heterogeneous substance B Homogeneous substance C Pure substances D Compound 1.2 The process whereby solid substances change to gaseous phase is called…….. (2) A Evaporation B Condensation C Sublimation D Melting 1.3 Which one of the following substance is a pure substance? (2) A Steel B Juice C Sulphur D Salt water 1.4 The number of neutrons of 24 12𝑀𝑔is: (2) A 6 B 24 C 36 D 12 1.5 Air cannot be called an element because: (2) A air can be separated into oxygen atoms B air is a pure substance C air contains more than one element D air can be liquefied. Page 3 of 11 Physical Sciences grade 10 P2 1.6 2014 June Examinations In which period of the periodic table is carbon found? A 14 B 2 C 4 D 6 1.7 The outer electron structure of a magnesium ion is exactly the same as: (2) A Sodium B Neon C Argon D Calcium 1.8 Which one of the following represents the electronic structure of phosphorus (P) (2) A 1s22s22p63s2 B 1s22s22p63s23p6 C 1s22s22p63s23p5 D 1s22s22p63s23p3 1.9 Ionic bonding occurs between: (2) A two non-metals B two metals C a metal and a non-metal. D a metal and a semi-metal. 1.10 If 30 g of reactant A reacts completely with 25 g of reactant B, which ONE of the (2) Following statements is CORRECT? A The total mass of products plus any unreacted reactants will be less than 55 g. B The total mass of products plus any unreacted reactants will be greater than 55 g. C The total mass of the products plus any unreacted reactants will be 55 g D The total mass of the products will be equal to 55 g (2) [20] Page 4 of 11 Physical Sciences grade 10 P2 2014 June Examinations QUESTION 2 The diagram below represent the heating curve for a certain substance Temperature (°C) 2. 100 Evaporation 0 melting time (s) -23 2.1 Define the (a) boiling point and(b) melting point of a substance. (4) 2.2 State the phase of the substance between -23°C and when it reaches 0°C. (2) 2.3 Give reason to your answer in 2.2.above. (2) 2.4 Name appropable substance that can be represented by the graph. (2) 2.5 State the reason why this substance has high boiling point. (2) 2.6 The substance takes longer to evaporate than to melt, give a reason for this. (2) [12] QUESTION 3 Mothibi and Mpumi place a solid compound on a heating plate and heat the substance uniformly at a constant rate. The table below shows the temperatures of the object at different times during the experiment: Time (minutes) 0 2 4 6 8 10 12 14 16 18 20 22 24 26 Temperature (C) 15,5 27 37 37 37 48 59 70 80 80 80 80 91 102 3.1 Suggest a suitable investigative question for this experiment. (2) Page 5 of 11 Physical Sciences grade 10 P2 2014 June Examinations 3.2 Suggest a suitable hypothesis for this experiment. (2) 3.3 Name ONE condition that must be kept constant to ensure a reliable result. (2) 3.4 Draw a graph of temperature versus time for the data observed above. (5) 3.5 What would you have observed in the beaker at 37 C? (2) 3.6 At what temperature does the compound melt? (2) 3.7 At what temperature does the compound boil? (1) 3.8 Provide a definition for the word “temperature”. (2) 3.9 Is this an example of a chemical or a physical change? (1) 3.10 From 16 to 18 minutes the temperature of the compound remains constant, despite the constant heating. Using your knowledge of intermolecular forces and temperature, explain what occurs between the particles of this substance as it changes phase. Be sure to explain why the temperature remains constant at certain points. (4) [23] QUESTION 4 4.1 4.2 4.3. Classify the following as Homogeneous, Heterogeneous or pure substances 4.1.1 Air we breath (1) 41.2 Sugar in water (1) 4.1.3 Copper metal (1) Write the names of the following substances: 4.2.1 KBr (2) 4.2.2 CaCO3 (2) 4.2.3 NH4Cℓ (2) Write the chemical formulae of the following substances and their atomic ratios 4.3.1 Zinc sulphate (2) 4.3.2 Potassium permanganate (2) 4.3.3 Hydrogen oxide (2) [17] Page 6 of 11 Physical Sciences grade 10 P2 2014 June Examinations QUESTION 5 5.1 The diagram below is the electron configuration of a neutral atom 3p ↓↑ ↓ ↓ 3s ↓↑ 2p ↓ ↑ ↓↑ ↓↑ 2s ↓↑ 1s ↓↑ Use the information given above to answer the questions that follows: 5.2. 5.1.1 What name is given to this type of diagram? (2) 5.1.2 Name the element represented by this electron configuration. (2) 5.1.3. Write the electron configuration of this element in the spdf… notation. (2) 5.1.4 State the group and period on the periodic table to which this element belongs (2) 5.1.5 Justify the answer to QUESTION 5.1.4 above. (2) 5.1.5 State whether the element is a metal or a non-metal. (1) Consider the electron dot structures of: X and · Y˸ ¨ 5.2.1 How many valence electrons are there in Y (1) 5.2.2 Draw the Lewis diagram for the product of X and Y (2) 5.2.3 Write down the symbols of X and Y (2) 5.2.4 If the atomic number of X is 11, write its name (1) [18] Page 7 of 11 Physical Sciences grade 10 P2 2014 June Examinations QUESTION 6 6.1 Complete the following table by writing the question numbers in your answer book. Write the appropriate answer next to each question number. You do not need to redraw the table. (7) Name of No. of electrons neutrons (a) (b) 12 11 23 (d) (e) (f) 36 17 (h) Z A Mg 12 Sodium ion (c) Chlorine atom Cℓ element Magnesium atom 6.2 No. of Symbol Naturally occurring chlorine consists of 76% Cℓ-35 and 24% Cℓ-37. Show, using a (3) suitable calculation, that the relative atomic mass of chlorine is 35,48. 6.3 What is the name given to two forms of the same element, such as Cℓ-35 and Cℓ-37? (2) [12] QUESTION 7 7.1 Sodium (Na) is in group I. What is the name of the products formed when potassium reacts with water? (2) 7.2 Write the balanced chemical equation for the reaction of magnesium with oxygen. (3) 7.3 Write the word and balanced chemical equations for the reaction of sodiumwith water. (5) 7.4 Sodium and potassium metals are always kept stored in oil. Why do you think that is? (2) 7.5 Neon(Ne) is an element found in group 18. Describe its chemical properties. (2) 7.6 What is the special name given to elements in group 18? (1) 7.7 Explain why elements in group 18 remained undiscovered for so long. (2) [16] QUESTION 8 8.1 Consider the reaction between sodium carbonate and hydrochloric acid. It reacts to produce sodium chloride, carbon dioxide and water. 8.1.1 What is the common name for sodium chloride? (1) Page 8 of 11 Physical Sciences grade 10 P2 8.1.2 Finish and balance the chemical equation below: Na2CO3 (s) + HCl (aq) 8.2 2014 June Examinations (3) 8.1.3 State the Law of Conservation of Matter. (3) 8.1.4 Show that the mass is conserved in the reaction. (6) Consider the production of hydrogen chloride. 8.2.1 Balance the equation below and fill in the missing volume of gaseous product (3) formed. H2 (g) 8.2.2 + Cl2 (g) HCl (g) If 8 cm3 of H2 reacts with an excess of Cl2, calculate how much of the HCl (g) is (5) produced. [21] QUESTION 9 In 1910, Ernest Rutherford directed positively charged radioactive particles into a thin sheet of gold to investigate what atoms were made of. He expected many of the particles to be deflected backwards, but noted that most of the particles passed straight through the sheet of gold, while only a few were deflected and bounced back. These results led to the further development of the atomic model. 9.1 Describe the model of the atom that the scientists believed was true before this experiment. Page 9 of 11 (3) Physical Sciences grade 10 P2 2014 June Examinations 9.2 Why did most of the particles pass straight through the thin sheet of gold? (2) 9.3 What caused some of the particles to be deflected? (2) 9.4 The results of this experiment led scientists to revise the structure of the atom. Refer to (3) THREE aspects of the atom to describe how the model was revised. 9.5 Use the scientific method to explain how this experiment helped scientists develop a (3) better understanding of the atom. [13] TOTAL SECTION B = 130 GRAND TOTAL = 150 Page 10 of 11 Physical Sciences grade 10 P2 2014 June Examinations Page 11 of 11