(EE) Teacher Key Work with Radicals and Integer Exponents Part 2
advertisement
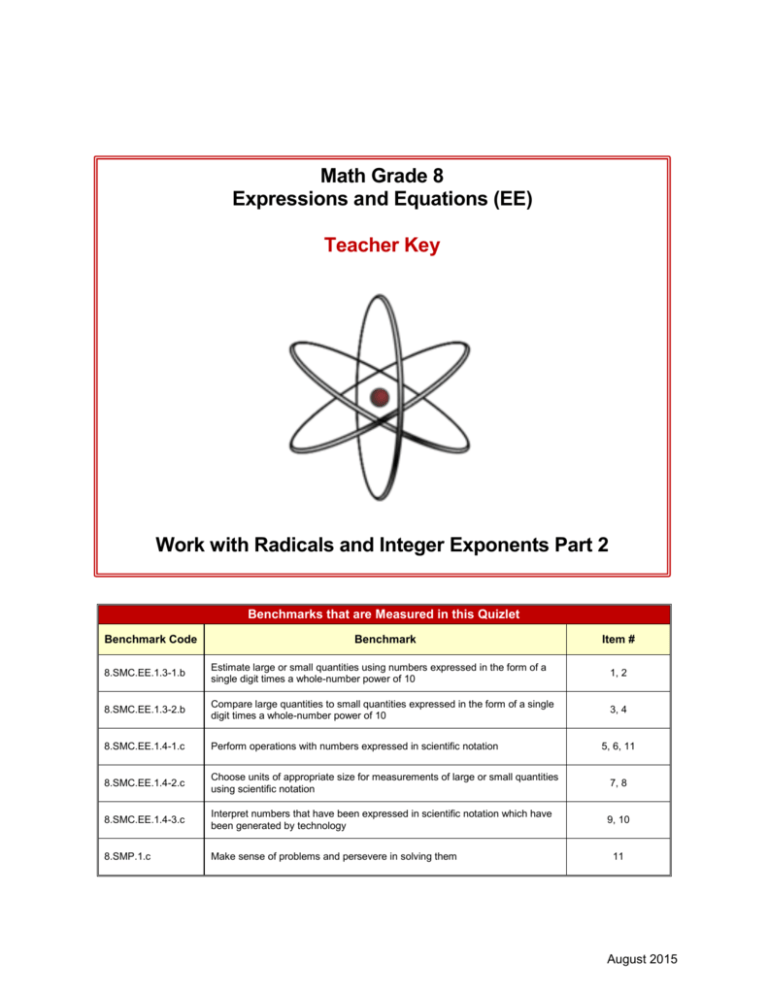
Math Grade 8 Expressions and Equations (EE) Teacher Key Work with Radicals and Integer Exponents Part 2 Benchmarks that are Measured in this Quizlet Benchmark Code Benchmark Item # 8.SMC.EE.1.3-1.b Estimate large or small quantities using numbers expressed in the form of a single digit times a whole-number power of 10 1, 2 8.SMC.EE.1.3-2.b Compare large quantities to small quantities expressed in the form of a single digit times a whole-number power of 10 3, 4 8.SMC.EE.1.4-1.c Perform operations with numbers expressed in scientific notation 8.SMC.EE.1.4-2.c Choose units of appropriate size for measurements of large or small quantities using scientific notation 7, 8 8.SMC.EE.1.4-3.c Interpret numbers that have been expressed in scientific notation which have been generated by technology 9, 10 8.SMP.1.c Make sense of problems and persevere in solving them 5, 6, 11 11 August 2015 MATH GR8 EE QUIZLET 2 Teacher Key NOTES TO TEACHERS This series of Quizlets is meant to be used for classroom formative assessment. Different from tools to evaluate student learning summatively, they are meant to be used by teachers as a part of the instructional process. Some features of these Quizlets are: The Quizlets are fully aligned with the Benchmarks. Each Quizlet is has a variety of item types: multiple choice (MC), multiple response (MR), short response (SR) and performance-based (PB). The Quizlets were created using Microsoft® Word so that they can be modified. Item stems and graphic organizers can be used to create additional assessments that are aligned to the benchmarks. Quizlets can be used to identify benchmarks where students are struggling or identify individual students who need additional learning opportunities. Item Teacher Key: Work with Radicals and Integer Exponents Part 2 Benchmark Code Benchmark Correct Answer Number of Points 1. 8.SMC.EE.1.3-1.b Estimate large or small quantities using numbers expressed in the form of a single digit times a whole-number power of 10 D 1 2. 8.SMC.EE.1.3-1.b Estimate large or small quantities using numbers expressed in the form of a single digit times a whole-number power of 10 SR 2 3. 8.SMC.EE.1.3-2.b Compare large quantities to small quantities expressed in the form of a single digit times a whole-number power of 10 C 1 4. 8.SMC.EE.1.3-2.b Compare large quantities to small quantities expressed in the form of a single digit times a whole-number power of 10 SR 2 5. 8.SMC.EE.1.4-1.c Perform operations with numbers expressed in scientific notation B 1 6. 8.SMC.EE.1.4-1.c Perform operations with numbers expressed in scientific notation SR 2 7. 8.SMC.EE.1.4-2.c Choose units of appropriate size for measurements of large or small quantities using scientific notation A 1 8. 8.SMC.EE.1.4-2.c Choose units of appropriate size for measurements of large or small quantities using scientific notation SR 2 Page 2 MATH GR8 EE QUIZLET 2 Teacher Key Item Teacher Key: Work with Radicals and Integer Exponents Part 2 Continued Benchmark Code Benchmark Correct Answer Number of Points 9. 8.SMC.EE.1.4-3.c Interpret numbers that have been expressed in scientific notation which have been generated by technology SR 2 10. 8.SMC.EE.1.4-3.c Interpret numbers that have been expressed in scientific notation which have been generated by technology SR 2 8.SMC.EE.1.4-1.c Perform operations with numbers expressed in scientific notation PB 8 11. 8.SMP.1.c Make sense of problems and persevere in solving them Page 3 MATH GR8 EE QUIZLET 2 Teacher Key Explained Answers SHORT RESPONSE EXPLAINED ANSWERS ITEM 2. Electron Mass (2 points) Benchmark: 8.SMC.EE.1.3-1.b Estimate large or small quantities using numbers expressed in the form of a single digit times a whole-number power of 10 Item Stem Responses The mass of an electron is 0.000548597 a.m.u. Express this number as a single digit times a whole number power of 10. 5 × 10–4 Points 2 points for correct response SHORT RESPONSE EXPLAINED ANSWERS ITEM 4. Compare Numbers (2 points) Benchmark: 8.SMC.EE.1.3-2.b Compare large quantities to small quantities expressed in the form of a single digit times a whole-number power of 10 Item Stem Responses Points −3 6 10 × 4 10−5 Given the numbers 6 × 10–3 and 4 × 10–5, how many times is the first number compared to the second number? 1.5 ×102 150 2 points for correct response SHORT RESPONSE EXPLAINED ANSWERS ITEM 6. Operations in Scientific Notation (2 points) Benchmark: 8.SMC.EE.1.4-1.c Perform operations with numbers expressed in scientific notation Item Stem Responses 4 10−3 × −6 1.6 10 What is (4 × 10–3) ÷ (1.6 × 10–6) ? 2.5 ×103 or 2500 Page 4 Points 2 points for correct response MATH GR8 EE QUIZLET 2 Teacher Key SHORT RESPONSE EXPLAINED ANSWERS ITEM 8. Ant Speed (2 points) Benchmark: 8.SMC.EE.1.4-2.c Choose units of appropriate size for measurements of large or small quantities using scientific notation Item Stem Responses 2 cm 1 km 3600 sec × × sec 100000 cm 1 hr Monica notices that an ant can move at a rate of 2 centimeters every second. How many kilometers per hour written in scientific notation, can the same ant move? 0.072 km/hr Points 2 points for correct response 7.2 × 10–2 km hr SHORT RESPONSE EXPLAINED ANSWERS ITEM 9. Galaxy Distance (2 points) Benchmark: 8.SMC.EE.1.4-3.c Interpret numbers that have been expressed in scientific notation which have been generated by technology Item Stem Responses 1017 The Canis Major Dwarf Galaxy is calculated to be 2.36 × km from the sun. The Large Magellanic Cloud is calculated to be 1.69 × 1018 km from the sun. Which distance is less? 2.36 × 1017 km is less Points 2 points for correct response SHORT RESPONSE EXPLAINED ANSWERS ITEM 10. Electron Proton Mass (2 points) Benchmark: 8.SMC.EE.1.4-3.c Interpret numbers that have been expressed in scientific notation which have been generated by technology Item Stem Responses The mass of an electron is calculated to be 9.1 × 10 –31 kg. The mass of a proton is calculated to be 1.7 × 10–27 kg. Which mass is greater? 1.7 × 10–27 kg is greater Page 5 Points 2 points for correct response MATH GR8 EE QUIZLET 2 Teacher Key PERFORMANCE-BASED RUBRIC ITEM 11. Atom Weight (8 points) Benchmark: 8.SMC.EE.1.4-1.c Perform operations with numbers expressed in scientific notation Benchmark: 8.SMP.1.c Make sense of problems and persevere in solving them Item Stem: Level Your chemistry teacher wants you to understand how weight is related to atoms. She gives you the following atomic weights from the periodic chart. 6.02 x 1023 Hydrogen (H) atoms weigh 1 gram 6.02 x 1023 Oxygen (O) atoms weigh 16 grams 6.02 x 1023 Carbon (C) atoms weigh 12 gram 6.02 x 1023 Nitrogen (N) atoms weigh 14 gram 4.0 (7-8 Points) 6.02 x 1023 Sodium (Na) atoms weigh 23 grams 3.0 6.02 x 1023 Chlorine (Cl) atoms weigh 35.5 grams Part A A molecule of water, H2O, contains 3 atoms (Note: O = O1) 6.02 x 1023 molecules of water, H2O, contains how many total atoms? What would it weigh? (5-6 Points) Part B You may know the formula of other molecules, if not make up any formula with the atoms above. Calculate the weight of your molecule and the number of atoms it contains. Part C Try 6.02 x 1023 molecules of Glucose sugar C6H12O6 Possible Responses: Part A 6.02 x 1023 molecules water, H2O, contains how many total atoms? What would it weigh? Atoms = 3 x 6.02 x 1023 = 18.06 x 1023 = 1.806 x 1024 2.0 (3-4 Points) It weighs 18 grams Criteria In addition to the understanding in Level 3.0, the student exhibits in-depth inferences and applications that go beyond what was taught. The student exhibits no major errors or omissions at eighth grade level by: 8.SMC.EE.1.4-1.c Performs operations with numbers expressed in scientific notation by multiplying them correctly and providing an answer with correct notation. 8.SMP.1.c Make sense of problems and persevere in solving them by correctly finding the weights of the molecules. The student exhibits major errors or omissions regarding the more complex ideas and processes, but makes no major errors or omissions regarding the simpler details and processes at eighth grade level, including: 8.SMC.EE.1.4-1.c Performs operations with numbers expressed in scientific notation by multiplying but not using correct notation. 8.SMP.1.c Make sense of problems and persevere in solving them by approximating the weight of the molecules. Part B (answers may vary) NaCl Atoms = 2 x 6.02 x 1023 = 12.04 x 1023 = 1.204 x 1024 1.0 It weighs 58.5 grams (1-2 Points) Part C 24 X 6.02 X 1023 = 144.4 X 1023 = 1.44 X 1025 It weighs 180 grams 0.0 (0 Points) Page 6 With help, the student demonstrates partial understanding of some of the score 2.0 elements and some of the score 3.0 elements at the eighth grade level. Even with help, the student demonstrates little understanding of skills.