Construction of alkBGTJL- and alkJ- expression vectors
advertisement

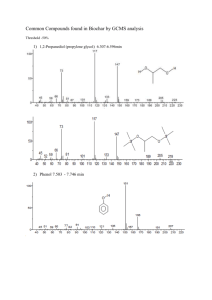
Reaction and catalyst engineering to exploit kinetically controlled whole-cell multistep biocatalysis for terminal FAME oxyfunctionalization Manfred Schrewe, Mattijs K. Julsing, Kerstin Lange, Eik Czarnotta, Andreas Schmid, and Bruno Bühler* Laboratory of Chemical Biotechnology, Department of Biochemical and Chemical Engineering, TU Dortmund University, Dortmund, Germany *Corresponding author. Mailing address: Laboratory of Chemical Biotechnology, Department of Biochemical and Chemical Engineering, TU Dortmund University, EmilFigge-Strasse 66, 44227 Dortmund, Germany. Phone: +49 231 755 7384. Fax: +49 231 755 7382. E-mail: bruno.buehler@bci.tu-dortmund.de Supporting Information Material and methods Strains and plasmids Strains and plasmids used in this study are listed in Table S1. E. coli DH5α was used for cloning purposes. For expression and biotransformation experiments, E. coli W3110 was transformed with different plasmids following standard procedures (Sambrook and Russell 2001). Table S1: Bacterial strains and plasmids used in this study Strain or Plasmid Characteristics Reference Strain fhuA2 (argF-lacZ)U169 phoA glnV44 80 (lacZ)M15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17 F- -1 rph-1 IN(rrnD-rrnE)1 (Hanahan 1983) pCOM10 alkane responsive broad-host-range vector; PalkB ; alkS (Smits et al. 2001) pGEc47 contains genes necessary for growth on alkanes (alkBFGHJKL and alkST) in the broad-host-range vector pLAFR1, Tcr blunt-end subcloning vector, Kmr (Eggink et al. 1987) E. coli DH5 E. coli W3110 (Bachmann 1987) Plasmid pSMART-HCKan pBT10 pBTL10 pBT10M contains the alkBGT genes inserted into the broad-host range vector pCOM10 contains alkL inserted into pBT10 Lucigen Corporation (Middleton, WI, USA) (Schrewe et al. 2011) (Julsing et al. 2012) this study pSMART-JKL MCS from pUC18 introduced into pBT10 downstream of alkG contains alkJKL in pSMART-HCKan pSMART-JL deletion of alkK in pSMART-JKL this study pBTJL10 contains alkJL in pBT10 this study pJ10 contains alkJ in broad-host range vector pCOM10 this study this study Construction of alkBGTJL- and alkJ- expression vectors Standard techniques were used for restriction analysis, cloning, and agarose gel electrophoresis (Sambrook and Russell 2001). Phusion High-Fidelity DNA Polymerase obtained from Finnzymes Oy (Espoo, Finland) was used for all PCR reactions. Restriction endonucleases were purchased from Fermentas GmbH (St. Leon-Rot, Germany) or New 1 England BioLabs GmbH (Frankfurt, Germany). All primers were purchased from Eurofins MWG (Ebersberg, Germany). Successful cloning was confirmed by sequencing by Eurofins MWG. Plasmid pBTJL10 was constructed based on the alkane responsive alkBGT-expression vector pBT10 (Table S1) (Schrewe et al. 2011). A multiple cloning site (MCS) from pUC18 (Yanisch-Perron et al. 1985) was introduced in the SalI restriction site of pBT10 downstream of alkG. For this purpose, the MCS from pUC18 was amplified via PCR using primers 1 and 2 (Table S2). The resulting PCR product was digested with XhoI and ligated into pBT10 cut with SalI (digestion with XhoI and SalI yields compatible ends). The orientation of the MCS was identified via sequencing and the resulting vector was designated pBT10M. The DNA fragment containing alkJKL (encoding the alcohol dehydrogenase, the acyl-CoA synthetase, and an outer membrane protein from P. putida GPo1) was amplified from pGEc47 (Eggink et al. 1987) via PCR using primers 3 and 4 (Table S2). Primer 3 contained a stop-codon in frame of the residual rest of alkH in pBT10 in order to prevent the formation of long non-sense gene-products. The PCR product was cloned into the blunt-end cloning vector pSMART-HCKan (Lucigen Corporation, Middleton, WI, USA) giving pSMART-JKL. Large parts of the alkK gene including the start-codon were deleted by digestion with BmgBI. BmgBI cuts three base pairs upstream of alkK and inside of alkK, resulting in the deletion of 1251 of 1644 base pairs of the gene. Subsequently, the vector was re-ligated and designated pSMART–JL. In order to obtain the final alkBGTJL-expression vector, the alkJL fragment was excised from pSMART-JL by digestion with XmaI and PspXI and ligated into pBT10M cut with XmaI and SalI (digestion with PspXI and SalI yields compatible ends), resulting in pBTJL10. The plasmid pJ10 was constructed based on the alkane responsive broad-host-range vector pCOM10 (Smits et al. 2001). The DNA fragment containing alkJ was amplified from 2 pBTJL10 via PCR using primers 5 and 6 (Table S2). The resulting PCR product was digested with EcoRI and SalI and ligated into pCOM10 cut with the same enzymes, resulting in pJ10 Table S2: PCR Primers used Entry Primer Sequence (5’ to 3’) Remark 1 MCS-f GCTACTCGAGATTACGAATTCGAGCTCGGTAC XhoI site underlined 2 MCS-r CAGTCTCGAGCCATATGCGGTGTGAAATAC XhoI site underlined 3 JKL-f CCCGGGTGAAAGGTGAAGCAGTTGATTGG 4 JKL-r GCTCGAGGGGGATGAGGAGCATTATTTG XmaI site underlined; stop-codon in bold PspXI site underlined 5 J-f CGCCGGAATTCATGTACGACTATATAATCGTTGGTGC EcoRI site underlined 6 J-r ACGCGTCGACTGAAACCGTCCGAA TCTATG SalI site underlined Determination of Partition Coefficients (KP) Partition coefficients were determined for aqueous-organic 2-LP-systems using the solvents BEHP, ethyl oleate, and dibutyl phthalate. Different concentrations of DAME (100– 1000 mM), HDAME (50–200 mM), and ODAME (25–100 mM) were added to the solvents, of which 1 mL was added to 1 mL KPi buffer in a pyrex tube. All samples were prepared in triplicates. The pyrex tubes were closed and incubated over night at 30°C and 400 rpm in an orbital shaker. Then, emulsions were transferred into 2 mL Eppendorf tubes and centrifuged at 17,000 x g for 5 min. The organic phase was diluted (1:100) with diethyl ether containing n-dodecane (0.2 mM) as internal standard and analyzed via GC. The aqueous phase was removed using a syringe, transferred to a new tube, and organic compounds were extracted with diethyl ether (1:1 ratio). After vortexing for 1 min, the Eppendorf tube was centrifuged at 17,000 x g and 4°C for 5 min and the organic phase was dried with anhydrous sodium sulphate, centrifuged, and transferred into GC-vials for analysis. 3 Results Apparent kinetics Figure S1 shows Michalis-Menten plots to investigate kinetics and determine the apparent kinetic parameters for the individual reaction steps catalysed by AlkBGT-containing cells. The experimental data was fitted using the program GraphPad Prism, Version 5.02 (GraphPad Software, Inc., La Jolla, USA). Figure S1: Apparent Michaelis-Menten-like kinetics for whole-cell conversions of DAME (A), HDAME (B), and ODAME (C) catalyzed by resting E. coli W3110 (pBTL10). Biomass concentrations in KPi buffer (50 mM, pH 7.4) containing 1% (w/v) glucose were 0.25, 0.18, and 0.28 g CDW L-1 for DAME, HDAME, and ODAME conversions, respectively. DAME, dodecanoic acid methyl ester; HDAME, 12-hydroxydodecanoic acid methyl ester, ODAME, 12-oxododecanoic acid methyl ester. Partition coefficients (KP) of DAME, HDAME, ODAME, and DDAME in 2LP systems with different carrier solvents Table S3 shows KP`s for DAME and its oxidation products with BEHP, ethyl oleate, dibutyl phthalate, and octanol (logKP,oct) as organic phases. As aqueous DAME concentrations were below the detection limit (~5 µM), 106 is given as a lower boundary for respective KP values. 4 Table S3: Partition coefficients of DAME and its oxygenation products in different organic/aqueous 2-LP systems without cells. BEHP Ethyl oleate Dibutyl phthalate DAME logPocta) 7.60 8.51 4.50 5.41 DAME > 106 > 106 > 106 - HDAME 2045 1987 2087 2314 ODAME 6035 5209 7419 5841 DDAME 18b) n.d. n.d. 15c) Performed in KPi buffer (50 mM, pH 7.4) containing 1% (w/v) glucose; n.d.: not determined; DAME, dodecanoic acid methyl ester; HDAME, 12-hydroxydodecanoic acid methyl ester, ODAME, 12-oxododecanoic acid methyl ester; DDAME, dodecanedioic acid monomethyl ester. a) values from www.chemspider.com b) obtained from a biotransformation using 25% (v/v) DAME in BEHP c) obtained from a biotransformation using DAME as organic phase (Co)-expression of alkJ Expression of AlkJ with different constructs was verified by SDS-PAGE analysis of crude cell extracts (Figure S2). Whereas prominent bands were found fof AlkB and AlkJ, AlkG and AlkT could not be visualized as described before (Schrewe et al. 2011). Similar AlkB levels were obtained with pBT10 and pBTJL10, whereas the latter construct gave an approximately 4-fold lower AlkJ level as compared to pJ10 Figure S2: SDS-PAGE (12%) of crude cell extracts from E. coli W3110 carrying pJ10 (containing alkJ), pBTJL10 (containing alkBGT and alkJ), or pCOM10 (control) harvested before (-) and 4 h after induction (+). PageRuler #26614 (Fermentas GmbH, St. Leon-Rot, Germany) was used as molecular weight marker. 5 Conversion of DAME, HDAME, and ODAME in resting-cell assays Figure S3: Oxygenation of DAME (A), HDAME (B), and ODAME (C) with resting E. coli W3110 (pBTL10) and oxidation of DAME (D), HDAME (E), and ODAME (F) with resting E. coli W3110 (pBTJL10). Biomass concentration: 0.95-1.0 gCDW L-1 in KPi buffer (50 mM, pH 7.4) containing 1% (w/v) glucose. 12Hydroxydodecanoic acid (HDA) hardly accumulated to concentrations above 0.01 mM and is added up with the HDAME concentration. DAME, dodecanoic acid methyl ester; HDAME, 12-hydroxydodecanoic acid methyl ester, ODAME, 12oxododecanoic acid methyl ester; DDAME, dodecanedioic acid monomethyl ester; DDA, dodecanedioic acid. 6 Pairwise addition of DAME, HDAME, ODAME, and DDAME in resting-cell assays Figure S4: Pairwise addition of DAME and HDAME (A), DAME and ODAME (B), HDAME and ODAME (C), DAME and DDAME (D), HDAME and DDAME (E), and ODAME and DDAME (F) to resting E. coli W3110 (pBTL10). Biomass concentration: 0.35 – 0.85 gCDW L-1 in KPi buffer (pH 7.4) containing 1% (w/v) glucose. 12-Hydroxydodecanoic acid (HAD) hardly accumulated to concentrations below 0.01 mM and is added up with the HDAME concentration. DAME, dodecanoic acid methyl ester; HDAME, 12-hydroxydodecanoic acid methyl ester, ODAME, 12oxododecanoic acid methyl ester; DDAME, dodecanedioic acid monomethyl ester; DDA, dodecanedioic acid. 7 Irreversible HDAME oxidation catalyzed by AlkJ Fig. S5: Influence of AlkJ on HDAME (A and C) and ODAME (B and D) conversion by resting cells. The compounds were added to E. coli W3110 (pCOM10) lacking AlkJ (A and B) and E. coli W3110 (pJ10) containing AlkJ (C and D). Biomass concentration: 1.0 gCDW L-1 in KPi buffer (pH 7.4) containing 1% (w/v) glucose. 12-Hydroxydodecanoic acid (HAD) and dodecanedioic acid (DDA) hardly accumulated to concentrations below 0.01 mM. HDAME, 12-hydroxydodecanoic acid methyl ester, ODAME, 12-oxododecanoic acid methyl ester; DDAME, dodecanedioic acid monomethyl ester. 8 References: Bachmann BJ. 1987. Derivatisations and genotypes of some mutant derivatives of Escherichia coli K-12. In: Neidhardt CF, editor. Escherichia coli and Salmonella typhimurium - Cellular and molecualar biology p1190-1219. Eggink G, Lageveen RG, Altenburg B, Witholt B. 1987. Controlled and functional expression of the Pseudomonas oleovorans alkane utilizing system in Pseudomonas putida and Escherichia coli. J. Biol. Chem. 262(36):17712-17718. Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166(4):557-580. Julsing MK, Schrewe M, Cornelissen S, Hermann I, Schmid A, Bühler B. 2012. Outer membrane protein AlkL boosts biocatalytic oxyfunctionalization of hydrophobic substrates in Escherichia coli. Appl. Environ. Microbiol. 78(16):5724-5733. Sambrook J, Russell DW. 2001. Molecular cloning - A laboratory manual. Cold Spring Harbor (NY): Cold Spring harbor Laboratory Press. Schrewe M, Magnusson AO, Willrodt C, Bühler B, Schmid A. 2011. Kinetic analysis of terminal and unactivated C-H bond oxyfunctionalization in fatty acid methyl esters by monooxygenase-based whole-cell biocatalysis. Adv. Synth. Catal. 353(18):3485-3495. Smits THM, Seeger MA, Witholt B, van Beilen JB. 2001. New alkane-responsive expression vectors for Escherichia coli and Pseudomonas. Plasmid 46(1):16-24. Yanisch-Perron C, Vieira J, Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33(1):103119. 9