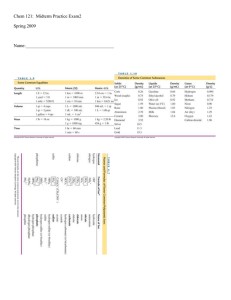
Chemistry Review Questions: Bonding, Gases, Liquids, Solutions
advertisement
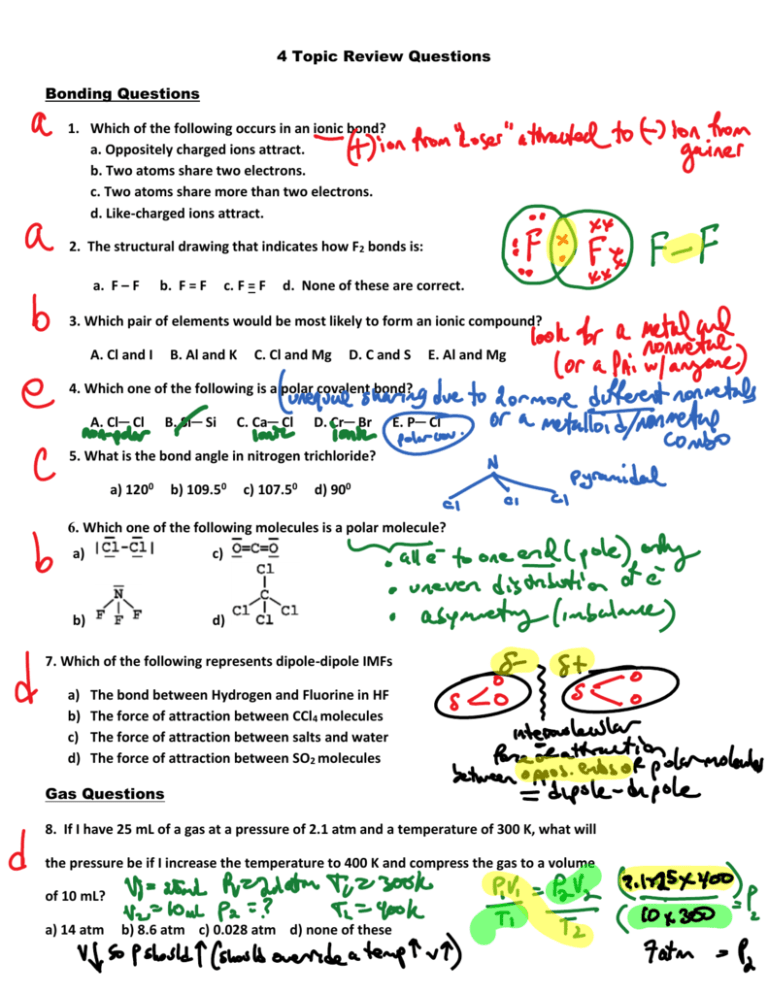
4 Topic Review Questions Bonding Questions 1. Which of the following occurs in an ionic bond? a. Oppositely charged ions attract. b. Two atoms share two electrons. c. Two atoms share more than two electrons. d. Like-charged ions attract. 2. The structural drawing that indicates how F2 bonds is: a. F – F b. F = F c. F = F d. None of these are correct. 3. Which pair of elements would be most likely to form an ionic compound? A. Cl and I B. Al and K C. Cl and Mg D. C and S E. Al and Mg 4. Which one of the following is a polar covalent bond? A. Cl─ Cl B. Si─ Si C. Ca─ Cl D. Cr─ Br E. P─ Cl 5. What is the bond angle in nitrogen trichloride? a) 1200 b) 109.50 c) 107.50 d) 900 6. Which one of the following molecules is a polar molecule? a) c) b) d) 7. Which of the following represents dipole-dipole IMFs a) b) c) d) The bond between Hydrogen and Fluorine in HF The force of attraction between CCl4 molecules The force of attraction between salts and water The force of attraction between SO2 molecules Gas Questions 8. If I have 25 mL of a gas at a pressure of 2.1 atm and a temperature of 300 K, what will the pressure be if I increase the temperature to 400 K and compress the gas to a volume of 10 mL? a) 14 atm b) 8.6 atm c) 0.028 atm d) none of these 9. What is the pressure exerted by some nitrogen gas collected in a tube filled with water on a day when the room temperature is 18.0 °C and the room pressure is 750.0 mmHg? [The partial pressure of water at 18 °C is 15.5 mmHg.] a) 15.5 mmHg d) 760.0 mmHg b) 750.0 mmHg e) 732.0 mmHg c) 734.5 mmHg 10. A sample of gas occupies 30,000 mL at 608torr and 25 C. How many moles of gas are in the sample? a) 22.4 b) 0.981 c) 1.02 d) 2.23 e) none of these 11. Given the decomposition of ammonia, How many liters of ammonia, measured at STP, are produced when 28.0 grams of nitrogen are completely consumed? (1) 5.60; (2) 11.2; (3) 22.4; (4) 44.8 Liquids and Phase Change Questions 12. 13. 14. 15. The vapor pressure of a liquid increases when: a) The temperature is raised c) The pressure is lowered b) The temperature is lowered d) none of these 16. The vapor pressure graph of an unknown liquid is shown below. Which of the following statements about this liquid is/are true? I. This liquid has weaker IMF’s than water. II. The liquid’s normal boiling point is around 75°C. III. The liquid boils at room temperature when the pressure is dropped to about 0.25 atm. a) II and III only d) I only b) II only c) I and III e) I, II, and III Solutions 17. If I have 30 grams of lithium hydroxide dissolved to make 3L of a solution, the molarity of this solution is: a) 0.42 M b) 1.26 M c) 10.0 M d) none of these 18. An unsaturated solution: a) Hasn’t dissolved as much solute as is theoretically possible b) Has dissolved exactly as much solute as is theoretically possible c) Is unstable because it has dissolved more solute than would be expected. d) none of these 19. Which would you expect to be more soluble in water at 00 C, sodium acetate or fluorine? a) sodium acetate b) fluorine c) it is impossible to tell 20. If I dilute 5 mL of 0.15 M NaCl to a final volume of 5 L, what’s the final concentration of NaCl? a) 0.00015 M b) 0.0015 M c) 15000 M d) none of these 21. What’s the molality if I have 5 L of a solution that contains 1.5 moles of lithium acetate? a) 1.5 m b) 3.33 m c) 0.30 m d) none of these 22. If 4.27 grams of sucrose, C12H22O11, are dissolved in 15.2 grams of water, what will be the boiling point of the resulting solution? (a) 101.64 oC (b) 100.42 oC (c) 99.626 oC (d) 100.73 oC (e) 101.42 oC