Preliminary Application Form
advertisement

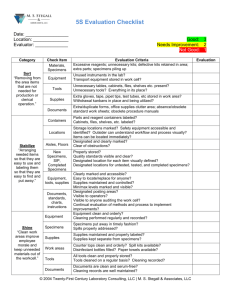
PLCO PAR-13-036 Preliminary Application Form A.1 DATE OF SUBMISSION: |__|__| / |__|__| / 20|__|__| Month Day Year A.3 PROJECT TITLE: _____________________________________________________________________________ A.4 CORRESPONDING INVESTIGATOR: ______________________________________ Affiliation Institution: Street: City: State B. ________________________________________ ________________________________________ ________________________________________ ___________________Zip: _________________ Phone: |__|__|__|-|__|__|__|-|__|__|__|__| Fax: E-mail: |__|__|__|-|__|__|__|-|__|__|__|__| _____________________________________ RESEARCH PROJECT INFORMATION: B. 1 STUDY SYNOPSIS: Provide a paragraph briefly describing your study and goals. B.2 SPECIFIC AIMS OF THE PROPOSAL: Provide one to two paragraphs describing the specific aims of the study, including the hypothesis to be tested C. WHAT IS YOUR STUDY POPULATION? (PRELIMINARY ESTIMATE): Provide as much detail as possible about your study population, its components and what defines each component. Example: 100 Total # of Cases: Example: 300 Total # of Controls: Example: Prostate cancer with biopsy Gleason score >7 What is your definition of a Case? Example: Men without any cancer What is your definition of a Control? Example: Age, time in storage, study year What are your matching criteria, if any? Example: 10% of the subjects randomly selected from the controls What QC samples will you include? D. WHAT ARE THE BIOLOGIC SPECIMENS REQUIRED? (PRELIMINARY ESTIMATE): Provide a listing of the specimens needed by completing the table below. Specimen Type Time from sample collection to diagnosis* (cases only) Desired Specimen Volume or Quantity Case Serum Less than two year 200ul Male Control Serum N/A 200ul Example: 100 Male case Any DNA Source (Buffy Coat or Whole Blood) Any 1ug Example: 300 Male control Any DNA Source (Buffy Coat or Whole Blood) Any 1ug Population Size Covariate (if any) Case/ Control Example: 100 Male Example: 300 Analytes free PSA, total PSA, intact PSA, hK2 free PSA, total PSA, intact PSA, hK2 E50 SNPs (details of the SNPs are indicated on attached table) 50 SNPs (details of the SNPs are indicated on attached table) *The PLCO blood specimens are collected prospectively at each screening visit (years 0-5). As such, the specimens are (generally) collected when subjects are asymptomatic and before they are diagnosed with (or worked up for a diagnosis of) cancer. These specimens are referred to as “pre-diagnostic”. In general, pre-diagnostic blood specimens are available from multiple time points for a given subject, and thus subjects generally have blood specimens with varying time intervals from collection to diagnosis. Therefore, when designing a study using PLCO blood specimens for biochemical analyses, investigators must consider the timing of the blood collection in relation to diagnosis, and choose the sample with the most appropriate timing that is consistent with the study aims. For example, for a study of early detection biomarkers for cancer, an investigator should consider using specimens closer to diagnosis, say within 1-2 years prior to diagnosis, when the tumor has developed but is still preclinical. For a study of etiologic biomarkers, on the other hand, an investigator should consider using specimens further away from diagnosis, say more than 3 years prior to diagnosis, when the subject is still at risk for developing, but has not developed, the tumor yet. Return completed form to zhucla@mail.nih.gov For Administrative Use Only Approved ☐ Disapproved ☐ Date |__||__| |__|__| |__|__|__|__| Month Day Year Study ID Number: |__|__|__|__| - |__|__|__|