The pH Scale
advertisement

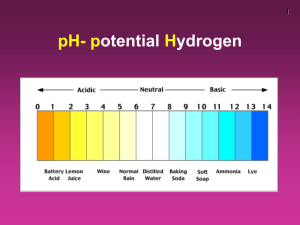
Name ____________________________________________ Date _________________________ Period__ Sample Problems for Chapter 14: Acids and Bases 14.6 The pH Scale (Read pgs. 458-464 in the chemistry textbook) 1. Locate and label each of the following on the pH scale below: a) neutral pH b) use an arrow to show the 'range' of increasingly acidic pH values c) use an arrow to show the 'range' of increasingly basic pH values d) draw a box around the pH that is 100X more basic than pH 10 e) draw a triangle around the the pH that is 1,000X more acidic than pH 9 f) underline the pH of hydrochloric acid g) draw a circle around the pH of 1.0M NaOH (lye) The pH Scale 1 2 3 4 5 6 7 8 9 10 11 12 13 14 2. Fill in the following table to the relationship of pH values to [H+] Solution Acidic [H+] pH Neural Basic 3. Identify the regions of the pH scale where: a) [H+] is increasing relative to neutral b) H+] is decreasing relative to neutral c) [OH-] is increasing relative to neutral d) [OH-]is decreasing relative to neutral The pH Scale 1 2 3 4 5 6 7 8 9 10 11 12 13 14 1 4. Consider the pH of the following items: Item root beer kitchen cleaner pickles glass cleaner cranberry juice pH 5.8 10.9 3.5 7.6 2.9 a) Place the pH values of the items on the list in order of most acidic to most basic. Most Most Acidic Basic b) Which item has the highest [H+]? ____________________ c) Which item has the highest [OH-]? ____________________ 5. If the pH of a solution reads 4.00, is the solution acidic / basic / neutral ? (circle one) 6. What does it mean when we say that the pH scale is a logarithmic scale? 2 [H+] is the antilog of the pH 6. Continued: 7. a) what is the [H+] of a solution with a pH of 2.00? Is this solution acidic or basic? _______________________ = __________ b) what is the [H+] of a solution with a pH of 2.50? Is this solution acidic or basic? ____________________ ________________________= __________ d) what is the [H+] of a solution with a pH of 12.0? Is this solution acidic or basic? ____________________ ________________________= __________ c) what is the [H+] of a solution with a pH of 8.30? Is this solution acidic or basic? ____________________ ____________________ ________________________ = __________ 3 e) what is the [H+] of a solution with a pH of 6.20? ____________________ Is this solution acidic or basic? _______________________ =__________ + 8. Conversely, if you already know the [H ] value, you can find out the pH by taking the log of the [H+]. This answer will be ( − ), so we multiply the answer by −1 to make the answer ( + ) pH is the negative log (base 10) of the [H+] concentration a) what is the pH of a solution with an [H+] of 1 x 10-6 M? Is this solution acidic or basic? _______________________ b) what is the pH of a solution with an [H+] of 1 x 10-10 M? Is this solution acidic or basic? _______________________ _______________________ c) what is the pH of a solution with an [H+] of 1.5 x 10-1 M? Is this solution acidic or basic? _______________________ ______________________ _______________________ d) what is the pH of a solution with an [H+] of 3.66 x 10-5 M? ______________________ Is this solution acidic or basic? _______________________ 4 e) what is the pH of a solution with an [H+] of 5 x 10-8 M? _______________________ Is this solution acidic or basic? _______________________ 9. If you know the [H+] it is easy to find the [OH-] concentration by remembering that KW = [H+] [OH-] and that the value of KW = 1.0 x 10-14 M (a) What is the [OH-] of a solution with an [H+] of 3.7 x 10-3? (b) What is the [OH-] of a solution with an [H+] of 5.5 x 10-13? (c) What is the [OH-] of a solution with an [H+] of 2.5 x 10-9? 10. Conversely, if you already know the [H+] you can use the KW to calculate the [OH-] value. (a) What is the [H+] of a solution with an [OH-] of 2 x 10-10? 5 (b) What is the [H+] of a solution with an [OH-] of 3.8 x 10-4? 12. What is the pOH scale? 13. The antilog of the [OH-] will give the pOH value. pOH is the negative log (base 10) of the OH-] concentration a) what is the pOH of a solution with an [OH-] of 1 x 10-6 M? _____________________ b) what is the pOH of a solution with an [OH-] of 1 x 10-10 M? _____________________ c) what is the pOH of a solution with an [OH-] of 1.5 x 10-1 M? ____________________ 14. The sum of the pH and the pOH will always equal 14 - so if you know either value you can quickly calculate the other value by subtracting: (a) What is the pH of a solution with a pOH of 10.0? (a) What is the pOH of a solution with a pH of 5.0? _______________________ _______________________ 6 (a) What is the pH of a solution with a pOH of 7.5? _______________________ 15. Why does a neutral solution have a pH of 7.00? 16. If you know the [OH-], how can you determine the pH of solution? STEP 1: STEP 2: 17. Calculate the pH of each solution given the following [H+] or [OH-] values: (a) [OH-] = 1.0 x 10-5 M STEP 1: _______________________ STEP 2: _______________________ (b) [OH-] = 2.5 x 10-11 STEP 1: _______________________ STEP 2: _______________________ (c) [H+] = 1.0 x 10-4 STEP 1: _______________________ (d) [OH-] = 8.2 x 10-4 STEP 1: _______________________ STEP 2: _______________________ 7 (e) [H+] = 3.6 x 10-9 STEP 1: _______________________ 18. A solution with a pH of 3 is 10 times more acidic than a solution with a pH of 4. Explain. 19. State whether each of the following solutions is acidic, basic, or neutral: __________ (a) soda, pH 3.22 __________ (b) laundry detergent, pOH 4.56 → 14.00 - 4.56 = 9.44 pH __________ (c) honey, pH 3.9 __________ (d) shampoo, pOH 8.3 → 14.00 - 8.3 = 5.70 pH __________ (e) rain, pH 5.8 __________ (f) cheese, pH 7.4 __________ (g) coffee, pH 5.52 __________ (h) drain cleaner, pOH 2.8 → 14.00 - 2.8 = 11.20 pH __________ (i) tomatoes, pH 4.2 8






![[H + ] [OH ] - CCBC Faculty Web](http://s2.studylib.net/store/data/005793401_1-b043355121eb738cc68e8c8b1b02be73-300x300.png)

