NEUROPATHOLOGY
NEUROPATHOLOGY
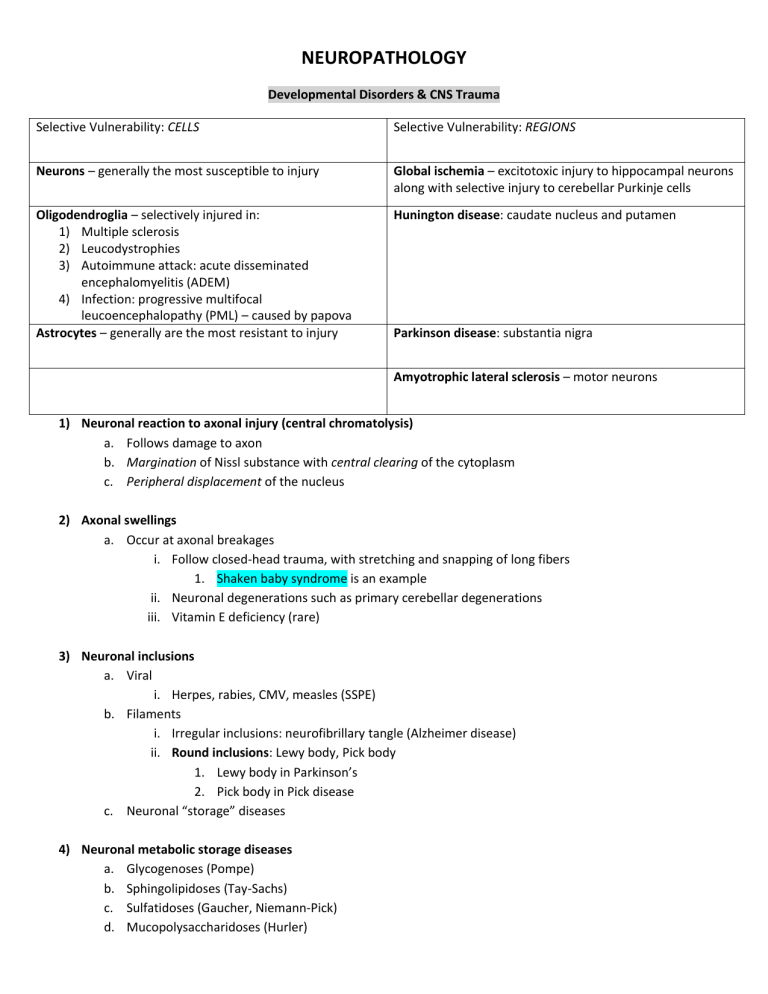
Developmental Disorders & CNS Trauma
Selective Vulnerability: REGIONS Selective Vulnerability: CELLS
Neurons – generally the most susceptible to injury Global ischemia – excitotoxic injury to hippocampal neurons along with selective injury to cerebellar Purkinje cells
Hunington disease: caudate nucleus and putamen Oligodendroglia – selectively injured in:
1) Multiple sclerosis
2) Leucodystrophies
3) Autoimmune attack: acute disseminated encephalomyelitis (ADEM)
4) Infection: progressive multifocal leucoencephalopathy (PML) – caused by papova
Astrocytes – generally are the most resistant to injury Parkinson disease: substantia nigra
Amyotrophic lateral sclerosis – motor neurons
1) Neuronal reaction to axonal injury (central chromatolysis) a.
Follows damage to axon b.
Margination of Nissl substance with central clearing of the cytoplasm c.
Peripheral displacement of the nucleus
2) Axonal swellings a.
Occur at axonal breakages i.
Follow closed-head trauma, with stretching and snapping of long fibers
1.
Shaken baby syndrome is an example ii.
Neuronal degenerations such as primary cerebellar degenerations iii.
Vitamin E deficiency (rare)
3) Neuronal inclusions a.
Viral i.
Herpes, rabies, CMV, measles (SSPE) b.
Filaments i.
Irregular inclusions: neurofibrillary tangle (Alzheimer disease) ii.
Round inclusions: Lewy body, Pick body
1.
Lewy body in Parkinson’s
2.
Pick body in Pick disease c.
Neuronal “storage” diseases
4) Neuronal metabolic storage diseases a.
Glycogenoses (Pompe) b.
Sphingolipidoses (Tay-Sachs) c.
Sulfatidoses (Gaucher, Niemann-Pick) d.
Mucopolysaccharidoses (Hurler)
5) Trans-synaptic degeneration of neurons a.
Secondary degeneration of a neuron connected to a dying neuron b.
Antegrade or retrograde c.
Primarily in “dedicated” tracts in CNS i.
Dedicated means 1on1 nerve interactions (classic example would be the optic neurons) d.
Potentially reversible
6) Oligodendroglial inclusions a.
Viral i.
PML – caused by papova virus b.
Filaments i.
Glial cell inclusions (GCIs) in some non-Alzheimer neurodegenerative diseases such as corticobasal degeneration, multiple system atrophy, and others
7) Astrocytic reactions a.
Gliosis: hypertrophy and hyperplasia with synthesis of glial filaments b.
Rosenthal fibers: swellings of processes with heat-shock proteins c.
“Alzheimer type II Glia” – seen in liver failure d.
Glial cytoplasmic inclusions e.
Elaboration of cytokines
8) Microglial reactions a.
Constitute in part the innate immune system of the brain (bone marrow derived cells) b.
CNS equivalents of macrophages c.
Respond to challenge with i.
Hypertrophy and hyperplasia ii.
Elaboration of cytokines iii.
phagocytosis d.
Also involved in synaptic remodeling
9) Ependymal reactions (cells which line the ventricles) a.
Ependymal “granulations” b.
Fusion of ependymal surfaces c.
Viral inclusions
By NEURONS
1)
2)
3)
Central chromatolysis
Inclusions
Trans-synaptic degeneration
Patterns of reaction to disease
By AXONS
1) Swellings
By GLIA
1) Inclusions
2) Gliosis
3) Immune responses
Pathophysiology of CNS Herniation
1) Anything that increases pressure inside the skull (hydrocephalus, brain swelling, mass lesion) will compress the brain and result in herniation
2) Types of herniation a.
Subfalcine herniation of cingulate gyrus b.
Transtentorial herniation of UNCUS i.
In extreme cases, the midbrain can be pushed against the contralateral tentorium resulting in necrosis
1.
This can create a false localizing sign with pathology on the opposite side of the body c.
Herniation of cerebellar tonsils through foramen magnum
3) Transtentorial herniation of the Uncus a.
Herniation palsy of the 3 rd cranial nerve i.
As the midbrain descends, the 3 rd cranial nerve is stretched over the edge of the tentorium ii.
This results in papillary dilation on the side of the mass lesion b.
Vascular consequences: i.
As the midbrain and pons descend, the basilar artery does not follow because it is ‘tethered’ above by the Circle of Willis ii.
The fine pontine penetrating arterioles are broken leading to pontine hemorrhage and death
4) Hydrocephalus a.
Marked dilation of the ventricular system due to obstruction of CSF flow b.
Causes of hydrocephalus i.
Occlusion of CSF flow
1.
Aqueduct of Sylvius (most common area creating hydrocephalus because it is the smallest hole)
2.
Arachnoid granulations – where CSF is reabsorbed at the superior sagittal sinus a.
Can occur during meningitis ii.
Increased CSF flow (rare) iii.
Compensatory to brain atrophy
Causes of congenital brain malformations:
Pediatric and Perinatal Neuropathology
Genetics:
1)
2)
Chromosomal (6%)
Single gene (2%)
Environmental:
1) Nutrition – folic acid deficient
2) Disease – diabetes
3) Toxins – alcohol, smoking
4) Infections – rubella, toxoplasmosis, CMV, syphilis
5) Radiation
Unknown (most cases**)
Embryology and Pathology of time periods
Time Period
7 days: Implantation
22-28 days: Neural
tube formation
1)
2)
Defects
Anencephaly – absence of brain and cranial vault
Spina bifida – bony defect in spine (most common) a) Posterior neuropore defect – cystic, skin-covered lesion on back may contain meningeal elements only (meningocele), or meningeal and neural elements
(myelomeningocele) i.
Myelomeningocele – absence of vertebral arches and herniation of meninges
4-8 weeks:
Organogensis
8 weeks-birth:
Migration of
neuroblasts
10 weeks: Susceptible to destructive lesions
Early = 10-25 weeks
Late = after 25 th week and malformed spinal cord into cystical lesion
3) Encephalocele – brain and ventricle stick out of hole that never closed in skull
4) Arnold-Chiari Malformation – hydrocephalus, widened foramen magnum, cerebella vermis herniation, ‘notch’ in cervical spinal cord a) Can survive and function normally
1) Holoprosencephaly – one large cerebral sphere instead of 2 hemispheres
2) Olfactory aplasia – absence of gyri recti
3) Agenesis of the corpus callosum – also has absence of cingulate gyrus
4) Dandy-Walker malformation - enlargement of the fourth ventricle, the space containing cerebrospinal fluid between the medulla and the cerebellum, a partial or complete absence of the cerebellar vermis – slow motor development and large heads
1) Agyria (no gyri) or Lissencephaly (smooth brain)
2) Pachygria (broad or coarse gyri) -
3) Heterotopias – the grey matter lining the lateral ventricle is abnormal
4) Polymicrogyria – note “busy” appearance of cortical gyral pattern a) Not compatible with normal intelligence b) The neuronal and molecular layers, but not the pia or meninges, wind up and down forming miniature pseudogyri within the true gyri c) Can also have Focal Polymicrogyria which is compatible with normal functioning
EARLY
1) Porencephaly – basically the same as schizencephaly, except that the destructive event has left a hole (‘pore’) a) Note abnormal gyri that ‘radiate’ out from the lesion
2) Schizencephaly –a destructive event (probably ischemic) has left symmetric clefts
(‘schisms’) in the brain
3) Hydrancephaly –
LATE
1) Germinal matrix hemorrhage – right next to ventricles
2) Choroid plexus hemorrhage
3) Parenchymal hemorrhage
4) Periventricular ‘leucomalacia’ – dead area in white matter
20 weeks on:
Myelination
Birth Trauma
Down Syndrome
1) Delayed myelination a) A response to almost any chronic insult b) No long-term sequelae i.
Myelination in the temporal lobes may be delayed until 2 nd decade
1) Tearing of tentorium
2) Linear tears of white matter
3) Tears of the spinal cord and cerebellar peduncles
1) Chromosomal defect
2) Small brain
3) Reduced dendritic complexity a) Displayed by Camera Lucida drawings
4) Premature Alzheimer changes (30s and 40s)
Other Perinatal Neuropathology
Pathology
Clinical signs
Types of diseases
Leucodystrophies
Myelin loss
Long tract signs:
1) Ataxia
2) Pyramidal signs
3) Sparing of subcortical U-fibers*
1) Metachromatic leucodystrophy a) Myelin breakdown products stain red-brown with cresyl violet stain
2) Krabbe’s (Globoid cell) leucodystrophy a) Perivascular macrophages are distended with myelin breakdown products
3) Adrenoleucodystrophy a) Later onset** (others occur early) b) Peroxisome defect**
Neuronal ‘storage’ diseases
Distended neurons
Grey matter signs:
1) Developmental delay
2) Mental retardation
3) Dementia
4) Seizures
CNS Trauma & Demyelinating Diseases
Learning Objectives:
1) Define the terms concussion and contusion a.
Concussion – transient functional impairment i.
No demonstratable anatomic abnormality ii.
Amnesia for the moment of injury b.
Contusion – focal necrosis of gyral crests i.
Sparing of sulci ii.
Old lesions are depressed and yellow (hemosiderin)
2) Define “contre-coup contusion” and explain its pathogenesis a.
Contre-coup contusions i.
Coup Lesions – at the site of trauma ii.
Contre-coup – opposite the site of trauma
1.
These usually result from falls from a standing position (direction of force pushes opposite side of brain against skull)
3) Internal vs. External injury a.
Diffuse axonal injury – results from angular acceleration within skull: shaking or oblique impacts i.
Involves long axon tracts of deep white matter, corpus callosum, cerebral peduncles, & brain stem ii.
Microscopically there are axonal swellings iii.
Present in 50% of patients who develop coma after trauma iv.
May explain persistent vegetative state in absence of gross lesions* v.
In milder form may explain concussion b.
Skull fractures – i.
Damage to vessels bleeding ii.
Damage to dura infection iii.
Damage to brain c.
Post-traumatic syndromes i.
Post-traumatic hydrocephalus ii.
Post-traumatic dementia: “punch-drunk” syndrome (dementia pugilistica) iii.
Post-traumatic epilepsy, meningioma, infection iv.
Post-traumatic psychiatric disorders
4) Define the pathogenic, anatomic, and clinical differences between epidural and subdural hematomas
Pathogenesis
Epidural Hematoma
Always associated with skull fracture
Subdural Hematoma
Trauma may be mild, without skull fracture
Anatomical damage Results from tearing of middle meningeal
artery
Tearing of meningeal bridging veins
Clinical Difference There may be a “lucid interval” of several hours May become chronic
1) Evolution: a) Lysis of clot (1 week) b) Early organization (2 weeks) c) Hylanized connective tissue (1-3months)
2) Often re-bleed, resulting in different stages of organization
Most common site*
5) Describe the gross and microscopical pathology of multiple sclerosis and of other demyelinating conditions a.
Multiple sclerosis i.
A disease of young adults (20-40) ii.
Irregular, but progressive course iii.
No constant clinical picture – relapsing iv.
Chronic Autoimmune destruction of myelin
1.
Lesions separated in “time and space” v.
Axonal preservation in MS vi.
Has acute and chronic pathological features b.
Acute disseminated encephalomyelitis i.
An acute, monophasic autoimmune disease with destruction of myelin
1.
No repeated or chronic attacks, people get better or die ii.
May follow viral infections or vaccines iii.
Fatal in 15-20% of cases
1.
Small hemorrhages begin to appear c.
Central Pontine myelinolysis i.
Formerly common in alcoholics ii.
Results from overly rapid correction of hyponatremia - IATROGENIC
6) Describe and explain the evolution of MS lesions a.
Axonal preservation occurs – if it was an infarct there would be loss of myelin and axons b.
Many of the oligodendrocytes disappear after the immune attack, but some hold on but don’t generate myelin i.
Remyelination does occur, but the axons have very thin myelin sheaths
1.
Never myelinates as good the 2 nd time aroun c.
The deficient during the initial attacks is worse than afterwards because of the inflammation i.
Acutely, the attack is full of inflammatory cells, edema, cytokines causing the inflammation ii.
There is still deficit afterwards b/c of loss of myelin, but there isn’t inflammation anymore d.
Macrophages come in and strip the myelin off e.
Lesions do not appear to attack specific anatomic boundaries, it just spreads out and attacks myelin f.
Chronic lesions have sclerotic astrocytes with limited macrophages i.
Bv is sclerotic and irregular ii.
Axons begin to disappear in chronic MS
7) Compare and contrast MS and acute disseminated encephalomyelitis
8) Explain the etiology of central pontine myelinolysis, and discuss prevention of this disease a.
This is due to a metabolic problem with serum sodium concentration b.
With the rapid correction of sodium in alcoholics comes the disease c.
Metabolites need to be added slowly over a period of time to prevent the disease
Cerebrovascular Diseases
Learning Objectives:
1) Define stroke, ischemia, hypoxia, and anoxia a.
Stroke (apoplexy) – i.
A transient ischemic attack (TIA) is a temporary (<24hrs) deficit due to temporarily decreased perfusion ii.
Caused by
1.
Brain infarction 80%
2.
Intracerebral hemorrhage 10%
3.
Subarachnoid hemorrhage 7%
4.
Misc 3% iii.
Stroke is the 3 rd leading cause of death in the elderly and the most prevalent neurological disorder b.
Ischemia – decrease or lack of blood c.
Hypoxia – decrease or lack of oxygen d.
Anoxia – no oxygen
2) List the different causes of stroke and discuss their relative prevalence, their gross and histological features, and their pathophysiology
Causes of Stroke
Brain Infarction
1) Thrombic
2) Embolic
Intracerebral hemorrhage
Due to hypertension
Prevalence Gross features
80% Acute (week 1)– softening, blurred grey-white margin, swelling with midline shifts
Subacute (weeks2&3) – tissue disintegrates, sharp demarcation of infracted area
Chronic (weeks 4+) – cavitation,
Gliosis (firm tissue)
Thrombosis @ carotid bifurcation is the most common
Embolism – most often from the heart
10% Bleeding into the basal ganglia, pons, and cerebellum
Pathophysiology
Thrombotic – caused by atherosclerosis
Embolic – caused by mural thrombi, valvular vegetations, fat emboli
Patterns of injury:
1) Focal infarction a.
Outcome is focal deficit
(classic stroke)
2) Focal patterns resulting from intermediate degrees of global ischemia a.
Hippocampal and cerebellar injury b.
Laminar necrosis c.
Watershed infarction
3) Global (entire brain) infarction a.
Ischemic/hypoxic encephalopathy (TIA or brain death)
1) From hypertension a.
Basal ganglia (common for hypertensive hemorrhages) b.
Pontine and cerebellar
2) From berry aneurysms a.
Massive subarachnoid hemorrhage
3) From vascular malformations a.
Arteriovenous malformation b.
Cavernous angioma c.
Venous malformation
Subarachnoid hemorrhage
Due to ruptured
berry aneurysm
7% Massive, clinically significant subarachnoid hemorrhage is almost always due to rupture of a
berry aneurysm **
Focal subarachnoid hemorrhage is common overlying contusions, infarcts, infectious foci
Berry (secular) aneurysm – involves
Circle of Willis, found in 2% of adults, etiology unknown
1) Aneurysm wall consists of vascular intima and adventitia, with absent smooth muscle and elastic
2) Warning symptoms due to leakage or nerve compression
(worst headache ever, vomiting, loss of consciousness)
3) Sudden death (25-50%)
4) Acute vasospasm leading to infarctions (and focal deficits)
5) Rebleeding occurs in survivors
Miscellaneous 3%
3) Describe the different types of vascular malformation in the CNS. Differentiate them according to types of component vessels and clinical signs and symptoms
Symptoms
Notes
Arteriovenous malformation
Component vessels Arteries and veins
Clinical signs Arterial pressure blood shunted into venous system veins dilate and sclerose in response
Abnormal veins prone to leakage, causing seizures and massive hemorrhages
Lesions are congenital, but: can be silent for many years, symptoms typically occur in young adults
Cavernous Angioma Venous Angioma
Veins
Abnormal, dilated and hyalinized veins arranged compactly with no intervening brain tissue
Can cause hemorrhage
Veins
Abnormal, dilated veins dispersed in brain tissue
DO NOT cause hemorrhage
NO intervening neural parenchyma
Throughout intervening brain tissue, do not generally bleed
Capillary telangiectasis
Capillaries
Dilated, but otherwise normal capillaries dispersed in normal brain tissue
Virtually never causes symptoms
Usually an incidental finding on autopsy
4) Describe the pathophysiological, histological, and clinical consequences of hypertension on the brain a.
Acute hypertension – medical emergency i.
Vessels begin to die, leakage, hemorrhage
1.
Pink necrosis called fibrinoid necrosis of the vessel walls ii.
Diffuse cerebral dysfunction b.
Chronic hypertension – more common i.
Cause slower changes that occur over years
ii.
Atherosclerosis occurs iii.
Arteriolar changes
1.
Arteriolar sclerosis
2.
Pulsating of vessels
3.
Microscopic hemorrhages lead to tiny cavities (lacunes)
4.
Microscopic aneurysms (Charcot-Bouchard) can lead to larger hemorrhages
5) Compare and contrast Binswanger disease and Leucoairosis a.
Binswanger disease i.
White matter infarcts* ii.
Destruction of white matter iii.
Clinical deficits iv.
Rare b.
Leucoairosis i.
Periventricular signal changes on CT, MRI ii.
Pathlogical significance unkown iii.
Clinical significance unknown iv.
Common in the elderly** v.
NOT INFARCTION
Infections of the CNS
1) List the different types of organisms that infect the CNS, and the common individual organisms within each of these groups a.
Bacteria – generally cause meningitis or abscess i.
Pneumococcus, mindingococcus, H. influenza, E. coli ii.
Signs and symptoms:
1.
Headache, photophobia, irritability, stupor, stiff neck, Ketnig’s sign iii.
Usually invades hematogenously or by direct extension from otitis or sinusitis iv.
CSF changes
1.
Cloudy or purulent fluid
2.
Increased pressure, WBC’s, protein
3.
Decreased sugar v.
Complications
1.
Acute thrombosis of inflamed meningeal vessels can lead to brain infarction
2.
Chronic scarring of meninges can lead to blockage of CSF flow and hydrocephalus
3.
Damage to cranial nevers can lead to blindness and cranial nerve palsies b.
Viruses i.
Acute encephalitis: arbovirus, herpes simplex, CMV, rabies ii.
Subacute encephalitis: HIV, JC virus (PML), measles (SSPE) iii.
Poliomyelitis: poliovirus, West Nile iv.
Relapsing: herpes zoster v.
Viral Tropism:
1.
Meninges – coxsackie, echo, mumps
2.
Temporal lobes – herpes simplex
3.
Dorsal ganglia – herpes zoster (varicella)
4.
Motor neurons – poliovirus, West Nile
5.
Cholinergic neurons – Rabies
6.
Oligodendrocytes – JC virus (PML) vi.
Viral ‘aseptic’ Meningitis
1.
More common than bacterial meningitis
2.
Echo, coxsackie, and other enteroviruses
3.
Benign: most patients recover
4.
CSF – increased lymphocytes rather than neutrophils, mildly increased protein (bacterial has increased protein), normal sugar (bacterial has decreased sugar), no bacteria vii.
Viral Encephalitis
1.
More seirous than viral meningitis a.
May be: i.
Endemic – arbovirus, poliomyelitis ii.
Sporadic – herpes simplex, CMV, rabies b.
Subacute forms also exist i.
HIV encephalitis ii.
PML – from JC virus iii.
SSPE – from measles
c.
Fungi – generally cause meningitis or abscess i.
Common Organisms - Candida, mucor, aspergillus, Cryptococcus
1.
Meningitis – cryptococcus
2.
Vascular involvement with brain infarcts – aspergillosis and mucor
3.
Encephalitis with microabscesses – Candida d.
Protozoans – toxoplasmosis, amoebae e.
Metazoans – cyestericosis f.
Prions – unique to CNS* i.
Pathology
1.
Spongiform change and amyloid plaques a.
Spongiform may be in grey or white matter b.
Plaques present in some diseases
2.
Almost complete lack of inflammatory response**
2) Describe the pathological and clinical features of each type of CNS infection a.
Acute Bacterial Cerebritis – transient stage leading to brain abscess i.
Can be hematogenous
1.
Most often from endocarditis
2.
Territory of MCA is most common site ii.
Can arise from local extension
1.
Paranasal sinusitis, mastoiditis iii.
CSF findings are mild and nonspecific
1.
LP may cause brain herniation (resulting form increased intercranial pressure) and death b.
Brain abscess i.
Complications
1.
Mass effect with herniation a.
Subfalcine (cingulated) b.
Transtentorial (uncus)
2.
Rupture into meninges with meningitis
3.
Rupture into ventricle with ventriculitis a.
The fibrous capsules of abscesses are thinner on the ventricular side, facilitating rupture inward c.
Chronic Bacterial Infections i.
Tuberculosis
1.
Meningoencephalitis (most common**) a.
Exudate over the base of brain, may spread via CSF b.
Hydrocephalus from arachnoid fibrosis c.
Infarcts from endarteritis d.
AIDS patients may contract M intracellulare with limited host response
2.
Tuberculoma a.
Intracerebral tuberculosis granuloma (‘gummas’) b.
May be associated with meningitis
3.
Spinal epidural granuloma (TB of the spine) a.
Mainly affects thoracic and lumbar vertebra b.
May cause compression fractures ii.
Neurosyphilis
1.
Occurs in 10% of untreated cases, years after primary infection
2.
3 main types involving: a.
Meninges –meningovascular syphilis (‘gummas’) b.
Cerebral cortex – causes mood alterations, often delusions of grandeur c.
Spinal cord – tabes dorsalis* i.
Degeneration of the posterior columns of spinal cord ii.
Atrophy and fibrosis of dorsal roots iii.
Neuroborreliosis (Lyme disease) d.
Arbovirus Encephalitis i.
Most common form of epidemic Encephalitis ii.
Outbreaks in late summer from airborne arthropod-borne infections iii.
WEST NILE has recently become important in US – can cause polio-like sequalae e.
West Nile virus i.
Viral illness with sequalae fever, headache, muscle aches, swollen lymph nodes, rash ii.
Acquired via mosquito bite iii.
Possible outcomes
1.
Complete recovery (>99%), death, survival with paralysis f.
Poliovirus i.
Clinically and pathologically related to West Nile ii.
Largely eradicated by childhood vaccination programs in the 1950’s iii.
A postpolio syndrome may affect survivors decades after infection
1.
Progressive weakness with muscle atrophy
2.
Pathogenesis unclear g.
Herpes simplex Encephalitis i.
Usually HSV-1 ii.
Severe disease, often fatal without treatment iii.
Preferentially involves area around Sylvian Fissure (temporal and frontal lobes) iv.
Necrotizing, with intranuclear inclusion bodies in both neurons and glia h.
Herpes zoster infection i.
Due to reactivation of varicella (chickenpox) virus latent in spinal or trigeminal ganglia ii.
Usually causes a painful vesicular skin infection with a distinct dermatome distribution (shingles)
iii.
More rarely causes a severe demyelinating Encephalitis in Immunocompromised patients that resembles PML i.
Rabies i.
Transmitted by bite of a rabid animal
1.
Almost ALL US cases are now due to BATS ii.
Local replication in muscle (except for bat rabies skin fibroblasts) iii.
Trans-synaptic retrograde spread along cholinergic pathways to CNS
1.
Prolonged incubation period (1-3months) means that post-exposure prophylaxis is feasible
(unique to rabies) iv.
Furious Rabies
1.
Fluctuating consciousness with periods of severe agitation, hyperactivity, confusion, and aggressiveness
2.
Phobic spasms (hydrophobia) v.
Dumb or Paralytic Rabies – occurs in Immunocompromised hosts j.
CMV ventriculoencephalitis i.
Fetal CMV: periventricular involvement with brain destruction
1.
Microcephaly
2.
Periventricular calcification ii.
Nuclear and cytoplasmic inclusions iii.
CMV also infects Immunocompromised adults, especially AIDS k.
HIV Encephalitis i.
CNS involved in as many as 60% of AIDS patients
1.
Leads to dementia (AIDS dementia complex)
2.
Vacuolar myelopathy a.
Resembles B12 deficiency b.
No virus in lesions
3.
AIDS myopathy a.
May resemble inflammatory myopathy ii.
Common opportunistic CNS infections in AIDS
1.
CMV Encephalitis
2.
Cerebral Toxoplasmosis
3.
Cryptoccocal meningitis
4.
Progressive multifocal leukoencephalopathy l.
Subacute viral infections i.
JC virus – infects Oligodendrocytes causing widespread demyelination
1.
Causes PML – WHITE MATTER INVOLVEMENT
2.
Primary, asymptomatic infection in most people
3.
Probably reactivation of virus in immunocompromised people (AIDS) ii.
Measles Virus – infects neurons and Oligodendrocytes
1.
Causes subacute sclerosing panencephalitis
2.
An altered persistent virus incapable of making complete virions
3.
May occur years after primary measles infection
m.
Cryptococcosis i.
Most common CNS fungal infection ii.
May be spontaneous or opportunistic
1.
Inhaled leading to lung infection iii.
Round organisms with thick mucoid capsule
1.
Mixed India Ink stain iv.
Minimal inflammatory response n.
Aspergillosis i.
Opportunistic – patients with profound neurtropenia (especially common in bone marrow transplant patients) ii.
Spread to brain may be: hematogenous from lungs or by direct extension from sinusitis iii.
Causes a vasculitis – invades vessel walls o.
Mucormycosis i.
Opportunistic – diabetic patients are particularly susceptible ii.
Mucor invades vessels and produces infarction iii.
May reach CNS by direct extension from nasopharynx p.
Candidiasis i.
Common complication of:
1.
Indwelling catheters
2.
Intensive chemotherapy and neutropenia ii.
Uncommon CNS problems with AIDS
1.
AIDS patients get candida (oral thrush) iii.
Microabscesses with polys iv.
Pseudohyphae – the infective form q.
Cerebral Toxoplasmosis i.
Protozoal infection ii.
In fetuses: T in TORCH iii.
In adults with AIDS r.
Cerebral Amebiasis i.
Acquired via the nose from swimming in warm, stagnant ponds s.
Cerebral Malaria t.
Cerebral Cysticercosis i.
Taenia solium (pork) u.
Cerebral Hydatidosis i.
Echinococcus granulosus (dog) v.
Kuru i.
“to shake with fear” ii.
Predominately a cerebellar disease
1.
Ataxia and tremor
2.
Dementia in terminal stages
iii.
Transmitted by canabalism iv.
Caused by PRIONS (infectious protein) w.
Creutzfedlt-Jacob disease i.
Sporadic CJD (most common CJD – usually 7 th decade) ii.
Familial CJD – germline PrP gene mutation (earlier onset) iii.
Iatrogenic CJD – infected grafts iv.
Variant (atypical) CJD – consumption of BSE beef
Neurodegenerative Diseases
Learning Objectives:
1) Define dementia; list the common causes of dementia a.
Dementia – a global loss of all higher mental functions i.
More than memory loss ii.
Different from mental retardation iii.
Memory loss and confusion are usually noted early iv.
Alzheimer disease is the most common cause of dementia b.
Causes of dementia: i.
Alzheimer ii.
Frontotemporal dementias (FTD(P)-17, Pick disease, Corticobasal degeneration, motor neuron disease-type) iii.
Vascular dementia iv.
Lewy body dementia v.
Hunington disease vi.
CJD vii.
Teritary syphilis viii.
Depression ix.
Metabolic (especially hypothyroidism) x.
AIDS dementia complex
2) Distinguish dementing diseases according to etiology, pathogensis, gross and histological findings, and clinical presentation a.
Alzheimer i.
The major clinical manifestation is dementia
1.
Slowly progressive with death after 5 years
2.
Late stage patients are bed-ridden and susceptible to pneumonia and sepsis ii.
Predominately a disease of AGING
1.
1% incidence at ages 60-64, rising to 40% at ages 85-89 iii.
Most cases (90%) are sporadic iv.
Familial (genetic) cases have earlier onset (age 50-60 or earlier) v.
Genetics
1.
Familial a.
Amyloid precursor protein (APP) b.
Presenilin 1 (14) c.
Presenilin 2 (1) vi.
Acquired Risk factors
1.
Age
2.
Head trauma
3.
Lifestyle: smoking, obesity, diet, hypertension, cholesterol, physical inactivity vii.
Pathogenesis
1.
Aβ amyloid deposition
2.
Hyperphosphorylated tau protein in neurons
3.
Microglial reaction to amyloid
4.
Microglial toxicity to neurons: cytokines and oxidative stress
5.
Vascular insufficiency
viii.
Pathology
1.
Aβ amyloid deposit in CORTEX (plaques) a.
Found throughout the cortex i.
Alpha-secretase necessary to properly digest amyloid into products that can be excreted ii.
If there is a problem with alpha-secretase, then β-secretase and γ-secretase take over and cause the amyloid to be dissociated into products that will cause Aβ deposits b.
Contain active microglia and astrocytes, damaged nerve processes, and dying neurons
2.
Aβ amyloid deposits in vessel walls (angiopathy)
3.
Neurofibrillary tangles a.
Begin to mesial temporal lobe; eventually spread throughout cortex b.
Insoluble aggregates of abnormal, Hyperphosphorylated tau protein in neurons b.
FTD(P)-17 Frontotemporal dementia with parkinsonism – chromosome 17 i.
Mutations in tau gene ii.
Dementia with parkinsonian symptoms iii.
Cerebral cortical atrophy with tau-containing neurofibrillary tangles, but not Aβ plaques iv.
Degeneration of substantia nigra c.
Pick Disease i.
Vary rare cause of dementia ii.
Usually, sporadic iii.
Well recognized because of its distinct pathology
1.
Striking atrophy “knife-edge” gyri a.
Severe atrophy of frontal lobes with sparring of parietal and occipital lobes
2.
Numerous Pick bodies in neurons: these are round, unlike neurofibrillary tangles d.
Progressive Supranuclear Palsy i.
Onset age 50-80, death within 5-7 years ii.
Clinical picture is dominated by difficulties with eye movement, speech, and a movement disorder resembling parkinsonism iii.
Dementia develops late in the disease iv.
Neuronal and glial tau pathology
1.
Tau neurofibrillary tangles in basal ganglia and brainstem
2.
Tauglial inclusions in basal ganglia and brainstem
3.
Involvement of the cerebral cortex is more limited
4.
There are no Aβ deposits e.
CJD f.
Tertiary syphilis g.
Depression h.
Metabolic (especially hypothyroidism) i.
AIDS dementia complex
3) Explain the role of microglia and cytokines in disease progression in Alzheimer disease
a.
Microglial reaction to amyloid b.
Microglial toxicity to neurons: cytokines and oxidative stress
4) Distinguish diseases involving movement disorders according to etiology, pathogenesis, gross and histological findings, and clinical presentation a.
Corticobasal degeneration i.
Movement disorder, often asymmetrical, and dementia ii.
Atrophy of motor cortex and of parietal cortex, also asymmetrical iii.
Pathology
1.
Ballooned neurons**
2.
Tau inclusions in glia and, less commonly, in neurons in affected cortex and in the brainstem – unilaterally* b.
Motor neuron disease-type i.
Small ubiquitin-positive neuronal inclusions with no other pathology ii.
May or may not have clinical motor neuron disease iii.
May or may not be genetic (familial)
1.
In familial cases some family members may have dementia, while other may have motor neuron disease c.
Vascular dementia i.
Generally caused by numerous small strokes ii.
Clinically distinguished by a “stepwise” progression, although this is not always reliable iii.
Synergistic with Alzheimer disease: less AD pathology is required for dementia if vascular disease is also present d.
Parkinson Disease i.
An idiopathic disease with distinctive Lewy bodies in the substantia nigra ii.
Parkinsonism – a clinical syndrome with many causes, most without Lewy bodies
1.
Caused by: a.
Drugs b.
1918 influenza c.
Multiple system atrophy** d.
Progressive supranuclear palsy** e.
Corticobasal degeneration ** iii.
Parkinson’s Clinically:
1.
Slowed movements and rigidity
2.
Tremor
3.
Preservation of higher cortical functions in most cases
4.
Some patients show psychiatric changes and progressive dementia: Dementia with Lewy iv.
Etiology bodies
1.
Most cases are sporadic
2.
Form familial cases, mutations have been found in genes for: a.
α-Synuclein: a presynaptic protein involved in regulation of dopamine transmission b.
Parkin: a protein involved in α-Synuclein degradation
v.
Pathology
1.
Degradation of dopaminergic pathways a.
Death of substantia nigra dopaminergic neurons b.
Loss of normal balance in basal ganglia circuits
2.
Loss of PIGMENT in substantia nigra** e.
Lewy body dementia i.
α-Synuclein immunochemistry led to recognition of DLB as a common disorder in the 1990’s
1.
DLB found in 15-25% of demented patients; many also have Alzheimer’s disease ii.
A dementing illness similar to Alzheimer disease, but often with hallucinations and fluctuation in symptoms iii.
There are always Lewy bodies in SN, but there is not always parkinsonism iv.
Overlap with Alzheimer disease: most (60-70%) DLB cases also show Alzheimer pathology f.
Multiple System Atrophy i.
Three formerly separate diseases, now united under the term MSA because of their common
α-Synuclein pathology
1.
Striatonigral degeneration (parkinsonian symptoms)
2.
Olivopontocerebellar atrophy (cerebellar dysfunction with ataxia)
3.
Shy-Drager syndrome (autonomic dysfunction) ii.
The common pathology is α-Synuclein inclusions in affected areas
1.
The inclusions are primarily in Oligodendrocytes g.
Huntington disease i.
Age of onset varies tremendously, but generally 30-50 ii.
Autosomal dominant: Huntington on chromosome 4 iii.
Large, involuntary, “dance-like” movements (chorea) are due to loss of regulation or cortical motor neurons iv.
Later, dementia v.
The gene can now be identified in prenatal screens
1.
CAG trinucleotide repeats in huntingtin gene a.
Impairs mitochondrial function and axonal transport b.
“Gain of Function” mutation c.
Anticipation occurs with CAG repeat expansion (worsening in successive generation) vi.
Pathology
1.
Degeneration of striate nuclei (caudate and putamen) a.
Loss of neurons: especially inhibitory GABA neurons b.
Leads to a disruption in excitation/inhibition balance in basal ganglia
2.
Later, cortical atrophy with loss of neurons h.
Spinocerebella ataxias i.
A group of diseases (all genetic): most are dominant, some recessive ii.
Some with trinucleotide repeats iii.
Degeneration of spinal and cerebellar neurons and tracts
1.
Friedreich ataxia & ataxia-telangietasia
a.
Friedreich ataxia i.
Begins in childhood; death within 5 years ii.
Associated with heart disease and diabetes iii.
GAA repeats in frataxin gene b.
ataxia-telangietasia i.
Begins in early childhood; death by age 20 ii.
Telangiectasias of conjunctiva, skin, and CNS iii.
Abnormal response to DNA damage*
Summary of Pathology: CELL TYPES WITH INCLUSIONS
Alzheimer disease
Pick disease
NEURONS
NEURONS
Progressive supranuclear Palsy NEURONS & GLIA
Corticobasal degeneration NEURONA & GLIA
Motor neuron disease
MND dementia
Parkinson disease
Multiple system atrophy
NEURONS
NEURONS
NEURONS
MOSTLY OLIGODENDROCYTES
Summary of Pathology:
DISTRIBUTION OF INCLUSIONS
Alzheimer disease CEREBRAL CORTEX & HIPPOCAMPUS
Pick disease CEREBRAL CORTEX & HIPPOCAMPUS
Progressive supranuclear Palsy BASAL GANGLIA & BRAINSTEM
Corticobasal degeneration
Motor neuron disease
MND dementia
Parkinson disease
Dem Lewy bodies
Multiple system atrophy
CEREBRAL CORTEX & BRAINSTEM
SC, BRAINSTEM
CEREBRAL CORTEX & HIPPOCAMPUS
BRAINSTEM (substantia nigra)
BRAINSTEM AND CORTEX
BRAINSTEM, SPINAL CORD, AUTONOMIC
GANGLIA
Morphology of inclusions
Large, flame shaped
Large, ROUND*
Large, flame shaped or small and irregular
Small and irregular
Small, round
Small, round
Small and irregular
Molecular pathology of inclusions
NFTs (AD, PSP)
Pick bodies
GCIs of CBD, PSP
GCIs of MSA
Lewy bodies (PD, DLB)
MND inclusions
Tau
++
++
++
α-synuclein
+
+ ubiquitin
+
+
+
+
++
5) Compare and contrast the various types of lecuodystrophy a.
Leucodystrophies – diffuse degeneration of CNS white matter due to malformed myelin b.
Clinical presentation is dominated by motor signs rather than cognitive decline: spasticity, hypotonia, ataxia c.
Most have onset in early childhood; an exception is ADRENOLEUCODYSTROPHY
d.
Types of Leucodystrophies: i.
Krabbe (globoid cell) lecuodystrophy
1.
Deficiency in galactocerebroside β-galactosidase
2.
Macrophages collect undigested cerebroside, and form multinucleated giant cells (“globoid
cells”) around blood vessels ii.
Metachromatic lecuodystrophy
1.
Arylsulfatase A deficiency a.
Defective degradation of sulfatides b.
Accumulated sulfatides stain red-brown with cresyl violet iii.
ADRENOLEUCODYSTROPHY
1.
Later onset (school-age childred ages 5-9) or (adult form)
2.
Peroxisome defect
3.
Adrenal involvement
4.
Inflammation
6) Describe the clinical and pathological features of the metabolic, nutritional, and toxic disorders covered in the lecture and in Robbins a.
Mitochondrial Diseases i.
These involve tissues with high aerobic demands: muscle, heart, retina, and brain ii.
Primarily diseases of young adults iii.
Variants include:
1.
MERRF, MELAS, Kearns-Sayre syndrome
2.
Leigh Disease a.
Fatal disease of early childhood b.
Caused by various mutations affecting cytochrome c oxidase c.
Resembles Wernicke-Korsakoff b.
Vitamin A deficiency: thiamine (B1) i.
May cause a peripheral neuropathy (beriberi) ii.
May cause degeneration of the mamillary bodies and brain tissue adjacent to CSF pathways that resembles Leigh disease iii.
W-K syndrome is most commonly seen in associate with cachexia or poor nutrition: alcoholism, GI disease, cancer, etc c.
Vitamin Deficiencies: B12 i.
Degradation of both ascending and descending tracts of spinal cord: subacute combined degeneration of the spinal cord ii.
Numbness and tingling of legs, proceeding to spastic weakness and paraplegia iii.
Folate deficiency can cause a similar syndrome d.
Metabolic disorders i.
Hypoglycemia – similar to hypoxic injury ii.
Hyperglycemia – dehydration affects brain function rapid rehydration can cause cerebral edema iii.
Hepatic encephalopathy – hyperammonenia leads to confusion, progressing to coma
1.
Can see altered astrocytes in the cerebral cortex
e.
Toxic Disorders i.
Carbon monoxide – similar to hypoxic injury
1.
Bilateral necrosis of globus pallidus ii.
Methanol – retinal degeneration, bilateral necrosis of putamen iii.
Ethanol – massive, acute ethanol intoxication iv.
Radiation – radionecrosis of brain is due to endothelial injury v.
Methotrexate + radiation injury
1.
White matter necrosis
2.
May occur months after exposure
Neoplastic Disease of the CNS
Learning Objectives:
1) List the various types of tumors that occur in the central and peripheral nervous system a.
Brain neoplasms are low frequency, but among the most common tumors in children i.
Location: Adults (70% Supratentorial) & Children (70% Infratentorial) b.
Etiology: i.
Radiation – meningiomas, sarcomas ii.
Immunosuppression - lymphoma iii.
Genetic Syndromes –
1.
Neurofibromatosis (many tumor types)
2.
Tuberous sclerosis (astrocytomas)
3.
Von-Hippel Lindau (hemangioblastomas) c.
Special Considerations: i.
Primary malignant tumors infiltrate the brain, while metastatic malignant tumors DO NOT ii.
Limited ability to resect infiltrating tumors iii.
Clinical course is critically dependent on anatomic location of tumor iv.
Primary brain tumors do NOT metastasize outside the CNS
Brain Neoplasms
Gliomas
1) Astrocytoma
2) Ependymoma
3) Oligodendroglioma
Neuronal
1) Ganglion cell tumors
‘Primative’
1) Medulloblastoma
Others
1) Meningioma (from
arachnoid cap cells)
2) Hemangioblastoma
(unknown
histogenesis)
3) Lymphoma
4) Metastatic tumors
Age Preferences in brain neoplasms
Infants ----- Children------------------------------------- Adults-------------------------------------------------- Elderly
Poorly differentiated tumors
Medulloblastoma
Pilocytic astrocytoma -------------------
Dysembryoplastic NET
Ependymoma -----------------------
Ganglion cell tumors --------------
Oligodendroglioma
Astrocytoma
Schwannoma
Central neurocytoma
Meningioma -----------------------------------------
Glioblastoma -----------
Lymphoma
Most common BRAIN TUMORS
In Adults:
1) Meningioma
2) Glioblastoma
3) Astrocytoma
4) Schwannoma (of C.N. VIII)
5) Lymphoma
In Children:
1) Medulloblastoma
2) Pilocytic Astrocytoma
3) Ependymoma
Grading of Neural Tumors
Most common SPINAL CORD TUMORS
Of Cord:
1) Astrocytoma
2) Ependymoma
Of Nerve Roots:
1) Meningioma
2) Schwannoma
Benign
1) Pilocytic Astrocytoma
2) Meningioma
3) Ganglion cell tumors
4) Dysembryoplastic NE tumor
5) Central neurocytoma
6) Schwannoma
7) Neurofibroma
Low Grade
1) Astrocytoma
2) Oligodendroglioma
3) Ependymoma
High Grade
1) Glioblastoma
2) CNS lymphoma
3) Medulloblastoma
4) Primitive NE tumor
5) Malignant peripheral nerve sheath tumor
2) For each of these, describe the histogenesis, gross appearance, microscopic appearance, clinical presentation, and degree of malignancy a.
Astrocytoma
i.
Malignant (fibrillary, or diffuse) astrocytomas
1.
Low grade astrocytoma (grade II), anaplastic astrocytoma (grade III), glioblastoma (grade
IV)
2.
Collectively account for 80% of all adult primary brain tumors
3.
Ages 30-60; higher grades at older ages
4.
Low grade astrocytomas may progress to higher grade with time
5.
Symptoms critically dependent on anatomic site
6.
Average survival a.
Low grade astrocytoma: 5 years b.
Glioblastoma: 8-10 months
A) Low grade Astrocytoma a.
Too many astrocytes and too much pleomorphism
B) Anaplastic astrocytoma a.
Intermediate grade with intermediate prognosis b.
Pathology shows still greater cellularity and nuclear pleomorphism, plus mitotic activity c.
Anaplastic astrocytomas generally receive post-operative therapy, unlike low-grade astrocytomas
C) Glioblastoma (high grade astrocytoma) a.
May arise from dedifferentiation of lower grade astrocytoma (more common in younger patients) or.. b.
May arise de novo (more common in older patients) c.
Histological features of anaplastic astrocytoma plus:
i.
2 NEW KEY features:
1.
Necrosis, due to tumor outgrowing its blood supply a.
The crowded cells around the necrosis (“pseudopalisading’) are migrating away from the ischemic focus b.
They are also expressing VEGF, which results in…
2.
Proliferating “balls” of small blood vessels (microvascular proliferation) responding to
VEGF secreted by ischemic tumor cells d.
**more cellularity, more pleomorphism, plus mitotic figures** ii.
Benign (special type) astrocytomas
(WHO grade I)
1.
Pilocytic astrocytoma
2.
Pleomorphic astrocytoma
3.
Subependymal giant cell astrocytomas of tuberous sclerosis
A) Pilocytic astrocytoma a.
Usually seen in children and usually in the cerebellum i.
Midline and associated CYST may cause mass effect b.
Also occur in hypothalamus, optic nerve, brainstem i.
Can cause seizures c.
2 key histological features: biphasic growth i.
Alternating fibrillar and loose areas ii.
ROSENTHAL fibers
B) Pleomorphic xanthoastrocytoma a.
Superficial, circumscribed, cerebral b.
Benign tumor of children and young adults c.
Striking tumor cell pleomorphism may falsely suggest a high-grade tumor d.
LIPID content (‘xantho’) is only seen in some examples
C) Subependymal giant cell astrocytoma a.
A component of tuberous sclerosis b.
Oligodendroglioma i.
5-15% of Gliomas ii.
Tumors of adults: 4 th -5 th decade iii.
Cerebral hemispheres; predilection for WHITE matter iv.
Slow growth v.
Prognosis relatively good vi.
Pathology
1.
Delicate branching vessels: “chicken wire pattern”
2.
Microcalcifications common
3.
“Fried egg” appearance a.
Sheets of uniform tumor cells, nuclei with finely granular chromatin surrounded by clear halos
c.
Neuronal Tumors i.
Ganglion cell tumors
1.
Generally benign
2.
Large, mature neurons may be the only feature (gangliocytoma) or may occur with a glial component (ganglioglioma) ii.
Central Neurocytoma
1.
Benign
2.
Intra- or periventricular lesions
3.
Composed of uniform small neurons d.
Dysembryoplastic Neuroepithelial tumor i.
A benign tumor of childhood ii.
Presents with seizures iii.
Multiple cortical nodules show diverse histological patterns iv.
A key pattern is normal neurons that “float” in a mucinous matrix between vessels lined by small round cells (SPECIFIC GLIONEURONAL COMPONENT) e.
Atypical teratoid/rhabdoid tumor i.
Uncommon, high grade tumor of very young adults
1.
-2% of childhood CNS tumors
2.
94% < 5 years of age
3.
Generally in posterior fossa ii.
Histologically the tumor shows many different lines of differentiation (teratoid) as well as cells with round accumulations of filaments (rhabdoid cells) f.
Ependymoma i.
Seen in both children and adults ii.
Clinical features depends on location
1.
Cells arranged around vessels, with thin ependymal processes directed towards vessel, create a RIBBONED appearance
2.
EM shows tiny lumens filled with microvilli and long serpentine cell junctions iii.
Slow growing iv.
Seeding of subarachnoid space in aggressive tumors v.
POOR prognosis (average survival 4 years) vi.
Locations
1.
Intracranial a.
Primarily the 4 th ventricle b.
Primarily the first 2 decades of life
2.
Intraspinal a.
Most common location in adults b.
Most frequent intraspinal glioma** vii.
Ependymoma of 4 th Ventricle
1.
Disseminates through the CSF
2.
Close proximity to pons and medulla makes complete resection impossible*
3.
Spinal cord examples are more easily reseceted*
g.
Other Ventricular Tumors i.
Subependymoma
1.
Benign, small nodules on ventricular surface a.
Small, button-like projection into ventricle, composed of a special cell type, the
subependymal astrocytes
2.
Origin from subependymal astrocytes (a special cell type)
3.
Usually incidental findings at autopsy ii.
Choroid plexus papilloma
1.
Can cause hydrocephalus in children
2.
Resembles normal choroid plexus iii.
Colloid cyst of 3 rd ventricle
1.
“ball valve” effect with intermittent hydrocephalus (intermittent headache, loss of consciousness)
2.
Can cause sudden death h.
Poorly Differentiated Tumors (all are tumors of childhood)
1.
ALL of these are known as PNETs (primitive neuroectodermal tumors) ii.
Medulloblastoma (cerebellum)
1.
Most common malignant brain tumor of children
2.
Solid tumor arising from cerebellar vermis
3.
Highly malignant, but sensitive to radiation and chemotherapy: 5-year survival is 75%
4.
Disseminates through the CSF pathways a.
Matastases to other parts of the CNS b.
“drop” metastases to cauda equine
5.
“Small blue cell” neoplasm a.
Extremelycellular b.
Abundant mitosis iii.
Neuroblastoma (cerebral hemispheres)
1.
Neuroblastic (primitive neuronal) differentiation iv.
Pineoblastoma (pineal region) v.
Retinoblastoma (eyes)
1.
Photoreceptor differentiation i.
Primary brain Lymphoma i.
A disease of the elderly ii.
Also a disease of immunosuppression iii.
Preference for periventicular areas iv.
Most are B-cell, large cell lymphomas v.
Tumor cells collect in perivascular spaces (perivascular cuffing, hooping) j.
Meningioma i.
Benign tumors derived from arachnoid cells ii.
Common locations
1.
Convexity meningiomas
2.
Skull base meningiomas iii.
Meningiomas do not invade brain, although they may invade the overlying skull
1.
May indent brain without invasion
2.
Invasion of bones is common, and does not indicate malignancy iv.
Meningioma Pathology
1.
Most meningiomas are ‘transitional’ types with whorls of spindled cells wrapped around more polygonal cells a.
There is often calcifications (psammoma bodies)
2.
There are numerous other histological types k.
Hemangioblastoma i.
Benign, vascular tumors of adults ii.
Most common in the cerebellum & often cystic iii.
May produce erythropoietin, causing erythrocytosis iv.
Cell of origin is unknown v.
Multiple hemangioblastomas are seen in von Hippel-Lindau syndrome l.
Metastatic Tumors i.
Most common origins of brain mets are LUNG, BREAST, SKIN (melanoma), KIDNEY, AND GI
1.
These are also the most common tumors ii.
Most frequent site is Grey-White junction in territory of MCA iii.
Mets are generally sharply demarcated (except melanoma) m.
Tumors of Nerve Roots and Peripheral Nerves i.
Schwannoma
1.
8 th cranial nerve
2.
Spinal nerve roots
3.
Peripheral nerves
4.
ALL are encapsulated lesions that do not infiltrate the parent nerve ii.
Neurofibroma
1.
Spinal roots (rare)
2.
Peripheral nerves
1)
2)
3)
4)
3.
Almost always part of neurofibromatosis
4.
DIFFUSE infiltration of the parent nerve iii.
Malignant (rare)
Schwannoma
Schwann cells
Adjacent to nerve
Encapsulated
Easily resectable without nerve damage
Neurofibroma
1) Schwann cells, neuritis, fibroblasts
2) Infiltrate nerve
3) Not encapsulated
4) Not resectable without sacrificing nerve
Review
:
A) Whorls
B) Psammoma bodies spindled cells wrapped around polygonal cells laminated calcium
C) “Fried-egg” cells
D) “Chicken-wire” vasculature small cells with central nuclei and clear cytoplasm branching small capillaries
E) Palisade alignment of tumor cells with each other
F) Pseudopalisading
G) True Rosette lining up of tumor cells around a central necrosis alignment of tumor cells around a central lumen or a central fibrillar area of cellular processes
H) Pseudorosette alignment of tumor cells around blood vessels
Pattern
Whorls, psammoma bodies
Fried-egg cells, chicken-wire vasculature
Pseudopalisades
True Palisades
Perivascular pseudorosettes
Meningioma
Oligodendroglioma
Glioblastoma
Schwannoma
Ependymoma
Tumor
3) Describe the common paraneoplastic syndromes that affect the nervous system a.
Paraneoplastic Syndromes i.
May involve central or peripheral nervous system ii.
Most common tumor: small cell carcinoma of lung iii.
Syndrome may precede clinical manifestations of malignant neoplasm b.
Types of Syndromes: i.
Lambert-Eaton myasthenic syndrome ii.
Stiff-man syndrome iii.
Retinal degeneration iv.
Limbic and brain stem encephalitis v.
Subacute cerebellar degeneration vi.
Subacute sensory neuropathy
4) Describe the clinical and pathological features of common familial tumor syndromes a.
Neurofibromatosis (von Recklinghausen disease) i.
Dominant inheritance ii.
Neurofibromatosis type 1
1.
Multiple peripheral nerve neurofibromas
2.
Pigmented nodules (Lisch) in iris
3.
Café-au-lait spots (melanosis) in skin
4.
Elephantiasis: increased connective tissue
5.
Increased incidence of malignant tumors iii.
Neurofibromatosis type 2
1.
Bilateral schwannomas of 8 th CN
2.
Increased incidence of meningiomas, gliomas b.
Tuberous Sclerosis i.
Dominant inheritance ii.
Hamartomas in brain and eyes
1.
Tubers in cerebral cortex (gliotic, malformed gyri)
2.
Subependymal giant cell astrocytoma
3.
Retinal flial hamartoma iii.
Angiofibromas of face (adenoma sebaceum) and other areas c.
Struge-Weber disease (encephalofacial angiomatosis) d.
Von hippel-Lindau disease i.
Dominant inheritance ii.
Multiple hemangioblastomas of cerebellum, retina, and spinal cord iii.
Erythrocytosis due to erythropoietin production iv.
Systemic findings
1.
Cysts in pancreas and kidneys & renal cell carcinoma & pheochromocytoma of adrenal
Diseases of Peripheral Nerves, Motor Neurons, and Muscle
Learning Objectives:
1) Distinguish the pathological features of primary axonal disease from those of primary demyelinating disease in peripheral nerves a.
There is normally a 2:1 ratio between axonal diameter and myelin sheath thickness b.
Disease can occur with SECONDARY demyelination because of axonal degeneration or through PRIMARY demyelination or a mix c.
Axonal Disease i.
Wallerian degeneration: when an axon is cut or damaged
1.
Collagen ‘pockets’ may form when Schwann cells have lost their axons and begin to encircle bunches of collagen fibers ii.
Distal Axonopathy or ‘dying back’ pattern: when a motor neuron is critically ill
1.
Macrophage-mediated demyelination: a macrophage surrounds a myelinated axon and begins to strip away the myelin
2.
Segmental demyelination: some axons have myelin while others do not
3.
Chronic demyelination and remyelination produces concentric circles of Schwann cells around axons (“onion bulbs”) a.
These are the most common in hereditary demyelinating diseases
2) Describe the clinical features, pathological features, and etiology of Guillain Barre syndrome, chronic inflammatory demyelinating poyradiculopathy, hereditary neuropathies, and diabetic neuropathy a.
There are INFECTIOUS causes of diseases of peripheral nerves including: i.
Leprosy
1.
Lepromatous leprosy a.
Schwann cell infection, demyelination, and axonal loss b.
Preferential involvement of pain fibers leads to unnoticed limb trauma
2.
Tuberculoid leprosy a.
Granulomas in small dermal nerves b.
Involvement is more focal ii.
Diphtheria iii.
Varicella-Zoster b.
Guillain Barre syndrome i.
An acute, life-threatening disease ii.
Rapidly ascending paralysis iii.
Autoimmune-mediated demyelinating disease often follows flu-like illness
1.
Focal loss of myelinated axons
2.
Can often be caused by campylobacter jejuni infection iv.
Respiratory support has reduced mortality c.
Chronic inflammatory demyelinating poyradiculopathy i.
A chronic, relapsing disease
1.
Very sybtle lymphocytic infiltrate in CIDR ii.
Also thought to be autoimmune-mediated iii.
Treatment with corticosteroids or plasmapheresis
d.
Hereditary neuropathies i.
Hereditary motor and sensory neuropathies (HMSNs)
1.
A large group of disorders, formerly known as Charcot-Marie-Tooth diseases
2.
Classified into 3 main groups a.
HMSN type I: b.
HMSN type II: c.
HMSN type III: demyelinating with onion bulbs
neuronal
genetically and morphologically diverse
3.
Generally mild diseases compatible with normal life span ii.
Others e.
Diabetic neuropathy i.
Very common: 50% of diabetics have neuropathy after 25 years of diabetes ii.
Sclerosis of intrafascicular arterioles (diabetic small vessel disease) iii.
Preferential involvement of small fibers, with loss of pain sensation with consequent distal limb ulcerations f.
Other common neuropathies i.
Uremic neuropathy ii.
Alcoholic neuropathy iii.
Amyloid neuropathy iv.
Various vitamin deficiencies v.
Long list of neurotoxic environmental toxins, especially lead and arsenic
3) Describe the clinical and pathological features of motor neuron disease (ALS) and of infantile spinal muscular atrophy (Werdnig-Hoffman disease) a.
Amyotrophic lateral sclerosis i.
Degeneration of motor systems
1.
Degeneration of corticospinal tracts
2.
Death of anterior horn motor neurons
3.
Atrophy of ventral nerve roots (and of muscle)
4.
More subtle changes in motor cortex ii.
Clinical correlates
1.
Slowly progressive, ascending paralysis
2.
Preservation of sensory modalities
3.
General preservation of higher cortical functions iii.
Pathogenesis of ALS
1.
Proposed pathogenic factors include a.
Deficiency of Vascular Endothelial Growth Factor (VEGF) b.
Oxidative stress from free radicals c.
Excitotoxicity d.
Neurofilament disorganization & microglial inflammation
2.
All of these factors might be explained by poor perfusion, with consequent generation of free radicals and oxidative stress, leading to susceptibility to excitotoxic input and cellular inability to maintain microfilament organization
3.
Many of these factors have been implicated in other neurodegenerative diseases as well
b.
Werdnig-Hoffman disease i.
Also known as Infantile Spinal Muscular Atrophy ii.
Presents as neonatal hypotonia (“floppy baby”) – which is not specific iii.
Death within first 1-2 years of life iv.
Mutation of SMN1 (Survival Motor Neuron 1) gene; mechanism of neuron death is unknown
4) Describe the clinical features and basic pathological patterns seen in: a.
Neurogenic and Neuromuscular Junction Diseases i.
Neurogenic atrophy
1.
2 types a.
Motor neuron disease b.
Peripheral neuropathies
2.
Early denervation – loss of motor units leads to: a.
Small angular fibers b.
Involvement of both fiber types
3.
Chronic denervation – axonal sprouting and reinnervation leads to: a.
Large motor units b.
Fiber type grouping
4.
Late denervation – loss of large motor units leads to: a.
Grouped atrophy ii.
Type 2 atrophy
1.
Seen with: diffuse, chronic disease, cachexia, corticosteroids b.
Myasthenia gravis i.
Autoantibodies against acetylcholine receptor ii.
Immunological destruction of neuromuscular junction
1.
Simplification of postsynaptic folds and compensatory proliferation of presynaptic vesicles iii.
Rapidly fading strength (‘myasthenia’) due to depletion of synaptic acetylcholine iv.
Thymic hyperplasia or thymoma v.
Associated with other autoimmune diseases c.
Inflammatory Myopathies
Polymyositis
Pathology Pathogenesis
Infrafascicular inflammation Cytotoxic T cells
Dermatomyositis
Extrafascicular inflammation; perifascicular atrophy
Humoral
Inclusion Body Myositis
Inclusions; rimmed vacuoles Degenerative
Clinical
Pain
Pain & rash
Steroid resistant
d.
Genetic Myopathies (dystrophic, congenital, and metabolic) i.
Muscular dystrophies
1.
Chronic, degenerative diseases a.
Duchenne MD i.
Deficiency of DYSTROPHIN
1.
‘delta’ lesions on myofiber necrosis seen on phase-contrast micro ii.
X-linked; high mutation rate iii.
Onset age 5-6, death in late teens or early 20s b.
Becker MD i.
A milder form of Duchenne ii.
Truncated dystrophin molecule due to Multiple-of-three base deletion c.
Myotonic MD i.
A tri-nucleotide repeat disease d.
Other, rarer forms i.
Deficiency of dystrophin-associated proteins ii.
Congenital myopathies
1.
Fixed, non-progressive deficits in muscle
2.
Weakness at birth, structural defects
3.
Many forms; most are named after their histological appearance a.
Centronuclear b.
Central core c.
Rod body d.
Etc. iii.
Metabolic myopathies
1.
Glycogen storage diseases a.
Phosphorylase deficiency (McArdle disease) i.
Mild disease; onset in adulthood ii.
Exercise intolerance, later weakness b.
Phosphofructokinase deficiency i.
Like McArdle c.
Acid maltase deficiency (Pompe disease) i.
Severe neonatal disease ii.
Floppy baby iii.
Death within 1-2 years iv.
Found in lysosomes
2.
Lipid storage diseases a.
Muscle Carnitine deficiency i.
A mild disease with generalized weakness b.
Carnitine palmityltransferase deficiency i.
Needed for fatty acid shuttle into the mitochondria c.
Systemic carnitine deficiency i.
A more severe disease with episodes of hepatic insufficiency and encephalopathy
3.
Mitochondrial defects a.
Abnormal mitochondria and “ragged red” fibers b.
May involve muscle, nerve, heart, retina, and/or brain; producing various syndromes c.
Common feature is abnormal mitochondria d.
“mitochondrial myopathy” – muscle weakness due to mitochondrial dysfunction e.
Critical Illness Myopathy i.
Acute loss of myosin thick filaments ii.
Occurs commonly in patients who are critically ill and respirator-dependent, especially following treatment with corticosteroids and with neuromuscular blocking agents iii.
Patients recover with time, assuming that their critical illness resolves iv.
Also called intensive care myopathy, thick filament myopathy, or acute quadriplegic myopathy v.
Loss of myosin filaments leads to loss of green staining on Gomori’s trichrome
Notes:
A) ‘Myopathic’ changes in muscle a.
Myofiber degeneration i.
Altered staining properties of fibers ii.
Vacuolization, macrophage invasion, etc b.
Myofiber regeneration i.
Central, enlarged nuclei ii.
Basophilia due to increased RNA content c.
Accompanying changes i.
Variation in fiber sizes ii.
Endomysial fibrosis with loss of polygonal outlines
B) Dystrophin the abnormal protein in Duchenne and Becker dystrophies, anchors the cytoskeleton to the
extracellular matrix through several dystrophin-associated glycoproteins (DAGs)
C) Causes of neonatal hypotonia a.
POOR prognosis i.
Infantile spinal muscular atrophy (Werdnig-Hoffman disease) ii.
Acid Maltase deficiency b.
BETTER prognosis i.
Congenital myopathies
Seizures and Epilepsy
Learning Objectives:
1) Recognize common seizure types a.
Seizure – clinical event associated with an abnormal, excessive, and hypersynchronous electrical discharge in a group of cortical neurons b.
Epilepsy – recurrent and unprovoked seizures i.
Epidemiology
1.
Approximately 1% of the population suffers from epilepsy
2.
About 2.5 mil patients in USA alone
3.
Cumulated adjusted lifetime risk 1.3-3.1%
4.
3 rd most common neurological disorder
5.
Occurs with increased excitation or reduced inhibition ii.
Etiology
1.
Cryptogenic (61%)
2.
Trauma
3.
Vascular (15%)
4.
Genetic a.
Inherited channelopathies (single-gene or complex inheritance)
5.
Metabolic
6.
Tumor c.
ILAE classification i.
Partial Seizures
1.
Simple partial seizures a.
Consciousness unimpaired
2.
Complex partial seizures a.
Consciousness impaired ii.
Generalized Seizures
1.
Primary generalized a.
Absence b.
JME
2.
Secondary generalized a.
Lennox-Gastaut d.
Temporal lobe seizures i.
Most common form of partial seizures ii.
Aura in 20-90% iii.
Commonly arins epigastric sensation iv.
Motionless stare v.
Orofacial or limb automatisms vi.
Prominent post-ictal confusion e.
Frontal lobe seizures i.
Frequently confused with non-epileptic events ii.
May occur in clusters iii.
Aura similar to temporal lobe seizures iv.
Bizarre, stereotypical movements including thrashing, bicycling, and tonic/dystonic posturing f.
Parietal lobe seizures i.
Sensorimotor involvement common ii.
Transient sensory symptoms
g.
Occipital lobe seizures i.
Uniformed visual hallucinations including flashing lights ii.
Ictal blindness iii.
Forced version of eyes and head h.
Neonatal seizures i.
Are almost ALWAYS FOCAL ii.
Are likely to involve temporal lobe iii.
Last 2-3 minutes iv.
Migrate v.
Are associated with a wide variety of ictal morphology
1.
Focal clonic
2.
Focal tonic
3.
myoclonic i.
Epilepsy syndrome during 1 st year of life i.
Infantile spasms ii.
Benign myoclonic epilepsy of infancy iii.
Severe myoclonic epilepsy of infancy iv.
Idiopathic or cryptogenic v.
Symptomatic
2) Identify role of various modalities in diagnosis of epilepsy a.
Imaging in Epilepsy i.
MRI superior to CT
1.
MRI yield in TLE a.
Hippocampal sclerosis 57.0% b.
Foreign tissue lesion 13.5% c.
Cortlical dysplasia 10.5% ii.
Special coronal sequences through medial temporal lobe, hippocampus, and amygdale in patients with intractable partial epilepsy iii.
FLAIR iv.
No imaging study required in idiopathic primary generalized epilepsy b.
Role of EEG in Epilepsy i.
Confirm diagnosis
1.
Yield of 1 st EEG for interictal abnormalities is 50-60%
2.
Serial EEG increases the yield to 92%
3.
Normal EEG is about 8% after serial EEGs a.
EEG abnormal in .5-3.5% of people without history of epilepsy ii.
Classify seizure type and epilepsy syndrome iii.
Guide therapy
1.
Prognosis
2.
Initiation of AED
3.
Discontinuing AEDs iv.
Non-convulsive status epilepticus c.
Monitoring Strategies i.
Synchronized video-EEG monitoring (surface electrodes) ii.
Intracranial recording
1.
Depth electrodes
2.
Subdural electrodes
3) Understand the rationale for treatment of epilepsy a.
Is epilepsy a progressive disorder? i.
Short-term problems
1.
Driving, work, psychosocial issues ii.
Long-term problems
1.
Memory loss, depression, AED adverse effects b.
Which drug and why? i.
Seizure type ii.
Etiology and EEG iii.
Patient characteristics iv.
Conventional vs new agents v.
Cost vi.
Appropriate 1 st line lagent will control seizures in 70% of patients vii.
Need to avoid sudden discontinuation of drugs as it may lead to withdrawal seizures viii.
Need to avoid side effects with slow titration and control serum levels c.
Single seizure (treat?) 70% recur into early epilepsy (monotherapy – 1 st line) 20-30% recur into chronic epilepsy (polytherapy with 1 st and 2 nd line drugs) 80% recur into Pharmaco-resistent epilepsy should now consider surgery or experimental drugs d.
Medical Intractability – inability to achieve acceptable seizures control despite adequate trials with a
sufficient number of drugs at does that are associated with no side effects or with acceptable side effects only e.
Refractory Epilepsy i.
Medications 40-60% seizure free ii.
Surgery 50-80% seizure free iii.
Vagal nerve stimulator iv.
Ketogenic diet
8-10% seizure free
30% seizure free
4) Understand the role of medical versus surgical therapy a.
Epilepsy Surgery i.
75,000 patients in USA with intractable epilepsy are possible surgical candidates ii.
Early surgery may prevent disabling cognitive impairment especially in children iii.
Early surgery also improves the chances of being seizure free post-op iv.
Candidates
1.
Medically intractable epilepsy
2.
Localized seizure focus such as mesial temporal sclerosis
3.
Cortical dysplasias
4.
Tumors v.
Contraindications
1.
Primary generalized epilepsies
2.
Progressive neurological disease
3.
Medical illness
4.
Psychosis vi.
Surgery Evaluation
1.
Positron emission tomography (PET)
2.
Ictal single photon emission tomography (SPECT)
3.
WADA (memory & language)
4.
f MIR
5.
MRS vii.
Results of Epilepsy Surgery
1.
Procedure: anterior temporal lobectomy and or amygdalohippocampectomy
2.
Indication: intractable epilepsy with a mesial temporal focus
3.
Seizure free: 66-68%
4.
Improved: 22%
b.
Vagus Nerve Stimulation i.
Indications
1.
Refractory epilepsy
2.
Respective surgery not an option
3.
Failed respective surgery ii.
Contraindications
1.
Cardiac or respiratory disease
2.
Non-epileptic seizures iii.
VNS Pulse Generator & Lead
1.
Pacemaker like pulse generator
2.
Bipolar lead with two stimulating electrodes
3.
Intermittent stimulation a.
30 seconds on/5 minutes off
4.
Mean decrease in seizure frequency 25-28% a.
8% were seizure free c.
Discontinuing Therapy i.
Consider discontinuing AED if seizure free for 2 or more years ii.
Taper over a 6 week to 3 month period iii.
MOST RELAPSES occur in the 1 st six months iv.
Remission in about 70% after 2 year seizure free period d.
Driving i.
33% report a seizure while driving ii.
55% had an accident while driving
Movement Disorders: diagnosis and treatment
Disorders – don’t forget that everything in lecture can be caused by drugs and can also be treated by drugs
HYPERKINETIC DISORDERS:
1) Essential Tremor/ Familial tremor a.
Disease MOST OFTEN misdiagnosed as Parkinson’s*** b.
Associated with activation of muscles – will go away with relaxation c.
Postural/kinetic d.
Treatment i.
Alcohol ii.
Beta-blockers iii.
Mysoline, Klonopin, iv.
Topamax (topirimate) – one of the better new drugs v.
Thalamic deep brain stimulation
2) Huntington’s Chorea a.
Autosomal dominant/C AG repeat i.
Average age of onset of symptoms is 40-42 (after onset, life span is about 15 years)
1.
Nowadays ii.
With increasing repeats, age of onset becomes younger and younger iii.
Huntington gene protein affects many pathways which makes target for treatment difficult b.
Clinical syndrome i.
Psychiatric ii.
Movement disorder iii.
Cognitive c.
Treatment – mood, anxiety, anti-psychotics i.
Antidepressants – manipulating serotonin ii.
Neuroleptics and atypical antipsychotics iii.
Monoamine depleting agents iv.
Benzodiazepines v.
Anti-epileptics for mood stabilization
3) Hemiballismus a.
Large amplitude, choreiform (one-sided, hence HEMI) b.
Subthalamic nucleus infarction c.
Usually self-limited d.
Treatment i.
Neuroleptics ii.
Monoamine depleting agents
4) Tourette’s Syndrome a.
Autosomal dominant with variable penetrance and sex dependence b.
Clinical syndrome i.
Disabling motor/vocal tics ii.
Onset before age 18 iii.
Duration > 1 year iv.
Idiopathic c.
Tics may be simple or complex (copralalia) i.
Needs a vocal tic* d.
Associated features i.
OCD & ADHD
e.
Treatment – YOU CAN TREAT WITHOUT GOING AFTER DOPAMINE i.
Neuroleptics ii.
Monoamine depletors iii.
Clonadine iv.
Klonopin
5) Myoclonus a.
Lightning-like interruption in normal muscle activity i.
Can be caused by many different drugs b.
Positive and/or negative i.
Positive – gain tone ii.
Negative – lose tone (will fall if you lose tone in lower extremeties) c.
Types: focal, multifocal, action, reflex, generalized i.
Acquired type the most common (often from cardiac arrests – ischemic damage to brain) d.
Treatment i.
Klonopin ii.
Mysoline iii.
Depakote iv.
Keppra – probably the best myoclonus drug
6) Dystonia a.
Involuntary contraction (e.g. writer’s cramp) b.
Types: focal, segmental, action, task-specific, generalized c.
Treatment i.
Anti-cholinergics ii.
Baclofen – central alpha-adrenergic agonist iii.
BOTOX – botulinum toxin
7) Restless Legs a.
A disorder characterized by an almost irresistible urge to move, usually associated with disagreeable leg sensations, worse during inactivity, and often interfering with sleep i.
More common in pregnancy because of iron deficiency ii.
Very stereotyped, repetitive movement almost always– more often in older adults b.
Frequently accompanied by Periodic Leg Movements of Sleep (PLMS) c.
Syndrome i.
Creepy, crawly, tingly ii.
Like worms or bugs crawling under the skin iii.
Painful, burning, or achy iv.
Like water running over the skin d.
Secondary RLS i.
Iron-deficiency anemia ii.
Uremia (20-40% of dialysis patients) iii.
Pregnancy (up to 27%) iv.
Neurological lesions
1.
Both spinal cord and peripheral nerve lesions v.
Drug-induced
1.
Tricyclics, SSRI’s, lithium, dopamine blockers e.
Treatment i.
Benzodiazepines ii.
Narcotics iii.
Dopamine agonists – for people with significant problems iv.
In the past, Levodopa was given (very short-lived) – symptoms just appear earlier in the day
HYPOKINETIC DISORDERS
Tauopathies
1) CBGB
2) PSP
Parkinsonian Syndromes
Synucleinopathies
Multiple System Atrophy
1) SND
2) SDS
3) OPCA
Lewy Body diseases
1) PD
2) PDD
3) DLB
1) Classification of Parkinson Syndromes in a community a.
Idiopathic PD – 85% of PS cases b.
Vascular Parkinson’s syndrome (multiple strokes) – 3% i.
Abrupt onset, usually unilateral ii.
Step-wise or no progression iii.
Other signs – hemiparesis, aphasia, hyperreflexia c.
Drug-induced parkinsonism (DIP) – 7-9% i.
Don’t really see this anymore because we’re using atypical anti-psychotics now d.
MSA (SDS, SND, OPCD) – 2.5% e.
PSP – 1.5% f.
CBGD – 0.5%
2) Increased Mortality with Parkinsonism: a.
Untreated IPD – average life span 7-9 years b.
Treated IPD – average life span 15-20 years i.
Life-span doubled after the advent of levodopa ii.
Dementia lowers life-span c.
Parkinson’s Plus Syndrome – average life span of 7-9 years i.
Mostly untreatable with medicine (dopaminergic stimulation) ii.
Early gait and balance problems
1.
Rapid progression, dysphagia, falling, older age at diagnosis, dementia
2.
Death caused by: aspiration pneumonia, autonomic failure, cancer iii.
Many different areas of the brain affected
1.
Shy-Drager Syndrome a.
Parkinsonism b.
Autonomic failure* i.
Drop in systolic and diastolic blood pressure c.
Urinary/sexual dysfunction i.
Persistent, involuntary partial or total bladder emptying ii.
Erectile dysfunction in males
2.
Multiple system Atrophy – Striatonigral Degeneration a.
Bradykinesia – very debilitating b.
Rigidity i.
People become locked inside their bodies and cannot move voluntarily c.
Postural instability d.
Tremor (postural, resting, or both) e.
Poor or no responsiveness to levodopa
3.
Progressive Supranuclear Palsy a.
Parkinsonism b.
Onset after 40, progressive c.
Supranuclear gaze palsy d.
Other i.
Dysarthria/dysphagia ii.
Falling early
4.
Corticobasal Ganglionic Degeneration a.
Parkinsonism b.
Cortical signs c.
Dystonia d.
Irregular action/postural tremor e.
Myoclonus f.
Alien limb
Diseases of the Peripheral Nervous System
Learning Objectives:
1) Diseases of PNS a.
Lower Motor Neuron – LMN i.
Anterior horn cell ii.
Root iii.
Nerve iv.
Neuromuscular junction v.
Muscle b.
CNS i.
Pyramidal tract (UMN) ii.
Cerebellum – coordination iii.
Basal ganglia – muscle tone, posture
Upper Motor Neuron
1) Increased tone (spastic)
2) Brisk reflexes
3) Plantars upgoing (babinski reflex)
4) No atrophy*
5) Weakness
6) weakness
Lower Motor Neuron
1) Reduced tone
2) Absent reflexes
3) Downgoing
4) Marked atrophy*
5) Fasiculations c.
Muscle Diseases i.
Involve larger muscles – proximal muscles of arms and legs ii.
Difficulty going up stairs, getting up from sitting position, combing hair iii.
Deep tendon reflexes normal until late stages d.
Inherited – long history, usually years, other family members affected e.
Acquired – short history, weeks or months, other family members not affected i.
Inflammatory – polymyositis, dematomyositis
1.
Polymyositis/ Dermatomyositis a.
Autoimmune disease of muscle b.
Painful or painless progressive proximal muscle weakness c.
There may be associated skin involvement with skin rash d.
The condition is then called dermatomyositis e.
Rarely cardiac muscle involved f.
Diagnosis: associated with underlying malignancy in adults > 60 i.
Elevated muscle enzymes - creatine phosphokinase (CPK), aldolase ii.
Biopsy shows muscle inflammation and necrosis iii.
Very good response to corticosteroids
ii.
Metabolic – endocrine disease a.
Thyroid Disease i.
Hyperthyroid myopathy
1.
Middle-aged men
2.
Marked wasting of muscle with weakness
3.
Hypercatabolic state with muscle breakdown
4.
Inflammation and infiltration of eye muscles and retroorbital tissues can occur with proptosis and restriction of eye movements
5.
Treatment of hyperthyroid state restores muscle function to normal ii.
Hypothyroid myopathy
1.
Can cause muscle weakness in children and adults
2.
Muscles stiff, bulky, and weak
3.
Muscle hypertrophy can occur in children
4.
Thyroid hormone replacement restores muscle function to normal b.
Adrenal disease i.
Excess of steroids from overactive adrenal glands (Cushing’s syndrome) or iatrogenic ii.
Iatrogenic cases likely with prolonged duration of therapy iii.
Fat deposition over face, abdomen with muscle weakness iv.
Treatment of adrenal over-activity or decrease in steroid dose reverses muscle weakness c.
Parathyroid disease
2.
Drugs a.
Focal myopathy i.
Pentazocine, Demerol, heroin b.
Generalized myopathy i.
Statins, procainamide, AZT, steroids, cyclosporine c.
Rhabdomyolysis i.
Cocaine, heroin, phencyclidine
3.
Alcohol
4.
Potassium a.
Muscle weakness can be associated with decrease or increase in serum K+ b.
Muscles become weak with fall in serum K < 3Meq/L (normal 3.5-5) i.
Increased to > 7 Meq/L of K+ usually occurs in renal failure
1.
High levels of K+ can cause cardiac arrest
2.
Hemodialysis is needed treatment c.
Loss of potassium can be due to GI or renal causes d.
GI causes include severe diarrhea, prolonged vomiting, laxative abuse e.
Renal causes include diuretic therapy f.
Replacement of serum potassium causes dramatic improvement in weakness iii.
Toxic – drugs such as statins iv.
Infectious – trichinosis
1.
Trichinosis is a parasitic infection acquired by ingestion of improperly cooked pork
2.
Caused by Trichinella Spiralis
3.
Larvae pass through intestinal wall into blood stream
4.
Carried to muscles where they cause inflammatory reaction with pain, weakness
5.
Arms and diaphragm more severely affected
6.
Treated with steroids and antiparasitic drugs
2) Inherited Muscles Diseases of the PNS a.
X-linked i.
Duchenne muscular dystrophy
1.
Males affected, onset in 3 rd year of life
2.
Proximal weakness of extremites and muscle hypertrophy
3.
Respiratory and cardiac muscle involved
4.
Wheelchair bound by age 10-12
5.
Pathogenesis a.
Marked by elevation of CPK b.
Encoded by gene Xp21 on chromosome X c.
Carriers and affected fetus diagnosed by genetic testing d.
Muscle lacks protein called dystrophin i.
Dystrophin stabilizes muscle membrane and its absence allows calcium mediated damage ii.
Beckers muscular dystrophy iii.
McArdles disease – inherited deficiency of the enzyme myophosphosphorylase
1.
The enzyme generates glucose from glycogen in active muscle
2.
With exercise enough glucose is not generated and muscle pain and cramps occur
3.
With vigorous exercise, muscle breakdown may occur b.
Autosomal Dominant i.
Myotonic dystrophy ii.
FSHD c.
Autosomal Recessive i.
Limb girdle dystrophy d.
Types: i.
Dystrophies ii.
Metabolic muscle disease iii.
Mitochondrial diseases
1.
Due to inherited defects in mitochondrial function
2.
Brain and skeletal muscle especially vulnerable in these cases
3.
Diseases called mitochondrial encephalomyopathies
4.
Muscle weakness associated with CNS symptoms like strokes, migraines, and seizures iv.
Neuromuscular Junction Diseases
1.
Myasthenia Gravis – autoimmune disease with circulating antibodies to acetylcholine receptor a.
Majority of patients have involvement of ocular and bulbar muscles b.
Symptoms include ptosis, diplopia, dysphagia, dysarthria c.
Weakness episodic, precipitated by exertion and relieved by rest d.
Later, limb and respiratory muscles involved e.
Sensations and deep tendon reflexes normal f.
Pathogenesis: i.
Circulating Abs to AChR ii.
Ab disrupts transmission at NMJ iii.
Thymus involved inproduction of antibodies to AChR iv.
90% of patients have abnormal thymus gland v.
70% have hyperplasia and 20% have thymomas g.
Diagnosis: i.
Measure receptor antibody in blood ii.
Positive in 60-80% of patients iii.
Image chest for thymic enlargement h.
Treatment: i.
Cholinesterase inhibitors – prolong life of Ach at NMJ ii.
Immunosuppressive agents & possible thymectomy (for invasive tumor)
i.
Drug Interactions in Myasthenia Gravis i.
Can unmask or worsen MG ii.
Do so usually by interfering with Ach release (presynaptic) iii.
May also interfere with action of Ach on the receptor (postsynaptic) e.
DRUGS i.
Antibiotics – gentamicin ii.
Cardiovascular drugs – beta blockers, calcium channel blockers, procainamide iii.
Psychotropic – phenothiazines iv.
Rheumatologic – d pencillamine v.
Anesthetic agents – NM blocking agents
3) Diseases of PNS a.
Polyneuropathy – symmetrical involvement of nerves in arms and legs i.
Signs and symptoms usually begin in arms and then spread to legs ii.
Distal involvement predominant – ‘glove and stocking’ distribution of deficit iii.
Deep tendon reflexes lost very early due to involvement of group 1a afferents of muscle spindle b.
Axonal Disease – involving primarily the axon: i.
Most neuropathies due to metabolic disease, toxins, deficiency states
1.
Metabolic – diabetes, renal failure, hypothyroidism
2.
Deficiency diseases – b12, b1, b6, niacin
3.
Drugs – vincristine, cisplatin, taxol
4.
Toxins – arsenic, lead
5.
Connective tissue disease – lupus, rheumatoid arthritis c.
Demyelinating disease – involving the myelin sheath i.
Inflammatory - Guillain barre syndrome ii.
Infection – HIV, Lyme disease, leprosy iii.
Paraneoplastic iv.
Inherited
1.
Pathogenesis: a.
Due to mutations in genes controlling peripheral myelin synthesis with formation of defective myelin b.
Long standing complaints c.
Skeletal abnormalities like pes cavus because of muscle weakness earl in life interferes with normal bone growth d.
Other family members affected, may have asymptomatic skeletal abnormalities e.
In some types, myelin unduly susceptible to pressure d.
Other inherited neuropathies due to excessive or abnormal deposition of proteins, lipids, etc. i.
Amyloid can be deposited in nerves and other organs like heart, kidneys – called AMYLOIDOSIS ii.
Affects smaller diameter unmyelinated fibers carry pain and temperature sensation and are part of the ANS iii.
Patients have pain and autonomic dysfunction iv.
In Refsums disease, phytanic acid accumulation in body causes involvement of nerves, eyes, skin, and heart e.
Mononeuropathy i.
Weakenss and sensory symptoms in distribution of a single nerve, eg. Carpal tunnel ii.
Causes:
1.
Compression – median nerve at wrist (carpal tunnel syndrome), ulnar nerve at elbow, peroneal nerve at knee
2.
Trauma – laceration, blunt injury
3.
Ischemia – vasculitis, diabetes
4.
Infiltration – sarcoidosis
5.
Infection – leprosy
f.
Root Disease - radiculopathy i.
Commonest cause is degenerative disc disease ii.
Most common at C5-6, 6-7 & L5-S1 iii.
Asymptomatic involvement – one arm or leg iv.
Root pains a characteristic feature v.
Motor and sensory defect in distribution of a specific root g.
Anterior Horn Cell Disease i.
Manifestations purely MOTOR ii.
Wasting prominent iii.
Prominent fasiculations iv.
Can be acute or chronic
1.
Poliomyelitis - acute a.
Viral infection of AHC b.
Virus travels by bloodstream to nervous system and localizes in AHC destroying them, causing acute flaccid weakness c.
Eradicated by effective immunization d.
West Nile virus now more common cause of viral AHC
2.
Lou Gehrigs (ALS) – chronic a.
Also called motor neuron disease b.
Degeneration of upper and lower motor neurons c.
Muscle atrophy, weakness, fasiculations, brisk reflexes, upgoing plantars d.
Also involves muscle of speech, swallowing, breathing h.
Investigations i.
Electromyography (EMG) & nerve conduction (NC) studies ii.
Nerve and muscle biopsy iii.
Muscle enzymes – creatine phosphokinase (CPK), aldolase iv.
Imaging of spine/roots – MRI v.
Laboratory tests – glucose, vitamin levels, HIV, lyme disase








