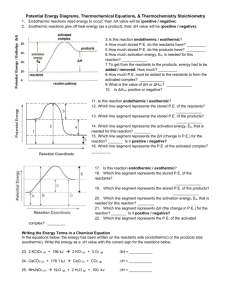
(g) ΔH rxn = − 92.2 kJ
advertisement

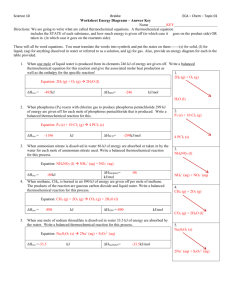
Name _________________________________________________ Date _______________ Period ____ Questions and Problems for 9.5: Energy in Chemical Reactions 9.5 Energy in Chemical Reactions (Read pgs. 269 - 273 in the chemistry textbook) 1. Define each of the following in terms of a chemical reaction: (a) system (b) surroundings (c) heat of reaction (d) enthalpy of reaction (ΔHrxn) (e) endothermic and how it relates to ΔHrxn (change of enthalpy of reaction) (f) exothermic and how it relates to ΔHrxn (change of enthalpy of reaction) (g) thermochemical equation 1 2. Determine if each of the following reactions is endothermic or exothermic: 3. (a) 2HgO(s) → 2 Hg(l) + O2(g) ΔHRXN = +185 kJ (b) N2(g) + 3H2(g) → 2NH3(g) ΔHRXN = −92.2 kJ ________________________ (c) CO2(g) + 2H2O(l) → CH4(g) + 2 O2(g) ΔHRXN = + 890 kJ ________________________ (d) CH4(g) + 2 O2(g) → CO2(g) + 2H2O(l) ΔHRXN = −890 kJ ________________________ ________________________ (a) If ΔHrxn is (−) energy was released and can be considered a product of the chemical reaction. (b) If ΔHrxn is (+) energy was absorbed and can be considered a product of the chemical reaction. (c) Using the position of the energy term, determine if each of the following reactions is endothermic or exothermic (i) C(s) + O2(g) → CO2(gl) + 393.5 kJ ________________________ (ii) O2(g) + 495.0 kJ → 2 O(g) ________________________ (iii) 1299.6 kJ + 2CO2(g) + H2O(l) → C2H2(g) + 5/2 O2(g) ________________________ (iv) CuO(s) + H2(g) → 129.7 kJ + Cu(s) + H2O(l) ________________________ (d) Insert the energy term as either a product or reactant based on the given ΔHrxn (i) CH4 (g) + 2 O2(g) → CO2(gl) + 2 H2O(l) ΔHrxn = −890 kJ (ii) 2 C(s) + H2(g) → C2H2(g) ΔHrxn = +226 kJ (iii) NO(g) + O3 (g) → NO2(g) + O2(g) ΔHrxn = −199 kJ (iv) CH3OH(g) → CO(g) + 2 H2(g) ΔHrxn = + 91 kJ 2 4. When it comes to determining ΔHrxn - REMEMBER: (a) REACTANT BOND BREAKING: (b) PRODUCT BOND MAKING: 5. What is the equation to determine ΔHrxn from ΔHreactants and ΔHproducts? 6. The reaction of 1 mole of glucose C6H12O6(s) with oxygen gas to produce carbon dioxide gas and liquid water is a combustion reaction and the energy of the products is LESS THAN the energy of the reactants by ≈ 2804 kJ (a) Write a balanced chemical equation for this combustion reaction. (b) It takes a total of 1273 kJ of energy to break the bonds of glucose and oxygen in this reaction. At the same time, 4076 kJ of energy are released by the formation of carbon dioxide and water. Is this reaction exothermic or endothermic? Explain (c) Calculate ΔHrxn in kilojoules for the combustion of glucose. 3 7. Methanol (CH3OH), which is used as a cooking fuel, undergoes combustion reaction. (a) Write a balanced chemical equation for this reaction. (b) 402.4 kJ of energy are required to combust the methanol. The formation of carbon dioxide and water releases 1932.3 kJ of energy. Calculate ΔHRXN for this reaction. (c) is this reaction exothermic or endothermic? Explain 8. In water solid sodium hydrogen carbonate will decompose into aqueous sodium hydroxide and carbon dioxide gas. (a) Write a balanced chemical equation for this decomposition reaction. (b) 948 kJ are required to break the bonds of the sodium hydrogen carbonate. The formation of the aqueous sodium hydroxide and the carbon dioxide releases 863 kJ of energy. Calculate ΔHRXN for this reaction. (c) is this reaction exothermic or endothermic? Explain 4 9. Answer the following: (a) In an exothermic reaction, is the energy of the products (Hproducts) less or greater than that of the reactants(Hreactants)? Explain. (b) In an endothermic reaction, is the energy of the products less or greater than that of the reactants? Explain. 10. When 2 moles of liquid water decomposes into its constituent elements, the energy of the products is 572 kJ more than the reactants. (a) Is this reaction exothermic or endothermic? Explain. (b) Write the balanced thermochemical equation for this decomposition reaction. 11. When nitrogen gas and oxygen gas form nitrogen monoxide gas the energy of the products is 90.2 kJ less than the energy of the reactants. (a) Is this reaction exothermic or endothermic? Explain. (b) Write the balanced thermochemical equation for this synthesis reaction. 5 12. When solid silicon and chlorine gas react to form gaseous silicon tetrachloride and the energy of the products is 657 kJ less than the energy of the reactants. (a) Is this reaction exothermic or endothermic? Explain. (a) Write the balanced thermochemical equation for this synthesis reaction. 13. Classify the following as exothermic or endothermic reactions: ____________________ (a) 550 kJ of heat is released ____________________ (b) The energy level of the products is higher than that of the reactants. ____________________ (c) The metabolism of glucose provides energy for the body. ____________________ (d) The energy level of the products is lower than that of the reactants. ____________________ (e) In the body, the synthesis of proteins requires energy. ____________________ (f) 125 kJ of energy is absorbed. 6 STOICHIOMETRY w/ ΔHrxn steps: 1. determine the ratio moles: ΔHrxn for the given chemical by using the coefficients of the balanced chemical equation 2. determine the molar mass of given chemical 3. calculate the moles of given chemical (if necessary) 4. calculate ΔHrxn for moles of given chemical 1. The thermochemical equation for the formation of ammonia from hydrogen and nitrogen is shown below. Calculate the heat, in kilojoules, released when 50.0 g of ammonia forms. N2(g) + 3 H2(g) → 2 NH3(g) ΔHrxn = − 92.2 kJ 7 2. Mercury (II) oxide decomposed to mercury and oxygen as shown below: 2 HgO(s) → 2 Hg(l) + O2(g) ΔHrxn = 182 kJ (a) Is this reaction exothermic or endothermic? (b) How many kJ are needed if 25.0 g of mercury (II) oxide react? 8 3. Sodium and chloride react to form sodium chloride as shown below: 2 Na(s) + Cl2(g) → 2 NaCl(s) ΔHrxn = − 819 kJ (a) Is this reaction exothermic or endothermic? (b) How many kJ are needed if 15.0 g of chlorine gas react? 9 4. Propane combusts as shown below: 2 C3H8 (g) + 5 O2(g) → 2 CO2(g) + 4 H2O(l) ΔHrxn = − 2220 kJ (a) Is this reaction exothermic or endothermic? (b) How many kJ are needed if 75.0 g of water are formed? 10