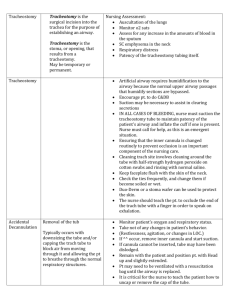
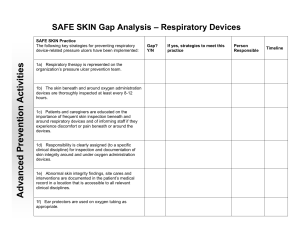
Secretion Extraction & Management and Respiratory
advertisement

Adult/Paediatric Suction Units and Respiratory Consumables for Secretion Management Assistive Technology Request Form 1. CONSUMER INFORMATION Medicare No: Last Name: First Name: Title: Mr Mrs Ms Date of birth: Miss Address: Postcode: Suburb: Phone: Mobile: Reason for request (tick one): New request Change in prescription N.B. Prescriptions older than 5 years will not be accepted, and an updated prescription/review will be requested. For repair, maintenance and replacements for existing EnableNSW consumers, please contact EnableNSW on the number listed below. 2. DIAGNOSIS Primary diagnosis: Date of Discharge from Hospital: 3. / / ELIGIBILITY FOR SUCTION UNIT (for clients with upper airway bypassed) The consumer’s upper airway is bypassed by means of surgical stoma, or artificial airway PLUS Consumer is unable to maintain their airway and independently clear secretions PLUS The consumer has trialed the device for 4 consecutive weeks, and has maintained a usage log. The trial log must document the number of suction episodes per day, including date, time and reason for suction episode (copy of log attached) Health Support Services – EnableNSW June 2011 Developed in collaboration with LTCSA & ACI – Respiratory Network Page 1 of 6 Adult/Paediatric Suction Units and Respiratory Consumables for Secretion Management Assistive Technology Request Form 4. ELIGIBILITY FOR SUCTION UNIT (for clients without a bypassed upper airway) The consumer does not have a bypassed upper airway PLUS Consumer is unable to maintain their airway and independently clear secretions PLUS The consumer has trialed the device for 12 weeks, and has maintained a usage log for a minimum of 4 consecutive weeks. The trial log must document the number of suction episodes per day, including date, time and reason for each suction episode (copy of log attached) 5. ELIGIBILITY FOR ELECTRICAL HUMIDIFICATION DEVICES Upper airway has been bypassed due to surgical stoma or artificial airway PLUS Consumer has sputum retention, plugging and secretions that require thinning PLUS Consumer uses invasive ventilation 6. ELIGIBILITY FOR TRACHEOSTOMY/LARYNGECTOMY TUBES, HME’S AND ATTACHMENTS Will the equipment be required on a permanent basis (≥ 12 months)? Yes No Is there a plan for decannulation within the next 12 months? Yes No For tracheostomy tubes, will the tube be changed in hospital (as an admitted inpatient)? Yes No Is the client and/or their carer educated in using the recommended equipment safely and Yes No Yes No Yes No Has a trial been completed? Yes No Are the consumables compatible with other equipment? Yes No appropriately, including, care and maintenance and troubleshooting? Is the consumer/carer aware of supply limits through EnableNSW and has information regarding purchase of additional supplies if required? Are any changes anticipated that may impact on this equipment request (e.g. anticipated size changes)? If Yes, please specify: 7. DEVICES REQUESTED – Once only supply Suction unit (electrical/portable) - 1 unit per consumer Manual suction unit (for consumers < 12 months of age) Heated humidifier (waterbath) for invasively ventilated consumers only Health Support Services – EnableNSW June 2011 Developed in collaboration with LTCSA & ACI – Respiratory Network Page 2 of 6 Adult/Paediatric Suction Units and Respiratory Consumables for Secretion Management Assistive Technology Request Form Type (as per contract): Current ventilator: Resuscitator – for consumers on life support ventilation only 10ml syringe for emergency cuff deflation (1 box only) Manometer – for cuffed tracheostomy Suction tubing (1 x 30m roll only) 8. CONSUMABLES REQUESTED Consumables may not be required or relevant for Supplier and Code: every application. (As per contract/s Annual Supply where relevant) Ventilation and Suction Consumables – recurrent supply Circuits Continuously ventilated (≥ 18 hours/day) Disposable: 26/yr Non-continuously ventilated (< 18 hours/day) Disposable: 12/yr Humidifier circuits for invasive ventilation Non-humidified ventilator circuits for invasive ventilation If using both non-humidified and humidified ventilator circuits, the annual supply for the combination will be arranged in discussion with EnableNSW Humidifier chamber – for invasive ventilation only Disposable: 26/yr Ventilator circuit accessories e.g. catheter mount, Disposable: 12/yr connectors, adaptors, PEEP valve etc. OR Non-Disposable: 3/yr Suction catheter 2160/yr Closed suction systems (letter of justification 120/yr attached) Tracheostomy Consumables – recurrent supply Health Support Services – EnableNSW June 2011 Developed in collaboration with LTCSA & ACI – Respiratory Network Page 3 of 6 Adult/Paediatric Suction Units and Respiratory Consumables for Secretion Management Assistive Technology Request Form Heat moisture exchangers 360/yr Tracheostomy tubes (cuffed or fenestrated tracheostomy Adults: 12/yr tube kit is inclusive of inner tube) Paed: 52/yr Additional tracheostomy inner tubes 2/yr Tracheostomy tube securing device Velcro tapes (neck strap) OR 20 /yr OR Cotton tape 1 Roll/yr Speaking Valves 3/yr Laryngectomy Consumables – recurrent supply Heat moisture exchange devices 360/yr OR Foam stoma covers HME Attachment devices Tracheostoma button OR 1/yr OR Standard adhesive seals (base plates) 360/yr Laryngectomy tubes (fenestrated or non-fenestrated) 1/yr Laryngectomy tube/Tracheostoma button securing device Neck straps OR Neck clip kit Health Support Services – EnableNSW June 2011 Developed in collaboration with LTCSA & ACI – Respiratory Network 12/yr OR 1/yr Page 4 of 6 Adult/Paediatric Suction Units and Respiratory Consumables for Secretion Management Assistive Technology Request Form 9. PLAN FOR IMPLEMENTATION Delivery address for equipment: Clients home address Other, provide details below: Name: Address: Phone: Fax: Please ensure the client has received information outlining the following: 10. - follow up clinical review arrangements - the clients ongoing compliance with therapy responsibilities - contact numbers for clinical advice regarding treatment and clinical care - client/carer has completed a Consumer Application Form PRESCRIBER DECLARATION Please provide the name, address and contact details of the clinician/prescriber Name: Address: Qualification/role: Provider Number: Phone: Fax: Email: DECLARATION I declare that I have assessed the consumer in consultation with an appropriate multidisciplinary team and have the required qualification and level of experience to prescribe this equipment according to the Professional Criteria for Prescribers. Signature: Date: Health Support Services – EnableNSW June 2011 Developed in collaboration with LTCSA & ACI – Respiratory Network Page 5 of 6 Adult/Paediatric Suction Units and Respiratory Consumables for Secretion Management Assistive Technology Request Form 11. OTHER CONTACTS Please provide the contact details of any other relevant health professionals who will continue to be involved with the management and monitoring of the client’s condition once in the community. The delegated professional(s) will be included in any correspondence regarding provisions to the client. Other Contact 1: Name: Address: Qualification/role: Provider Number: Phone: Fax: Email: Other Contact 2: Name: Address: Qualification/role: Provider Number: Phone: Fax: Email: EnableNSW contact details Email: enable@hss.health.nsw.gov.au Post: EnableNSW Health Support Services Locked Bag 5270 PARRAMATTA NSW 2124 Fax: (02) 8797 6543 If you require assistance or further information to complete this form please contact EnableNSW at 1800 ENABLE (1800 362 253). Health Support Services – EnableNSW June 2011 Developed in collaboration with LTCSA & ACI – Respiratory Network Page 6 of 6