Experiment 31 - richardkesslerhfa
advertisement

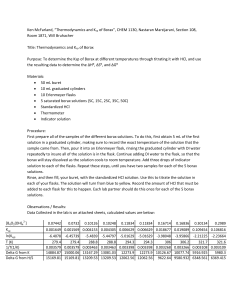
A goal of every AP chemistry instructor should be to develop a deeper understanding and appreciation of the important aspects and skills of thermo experiments. Scaffolding your activities, such as from a simple calorimetry experiment such as the Handwarmer design in the Guided Inquiry Experiments Manual to a second investigation utilizing Hess's Law. like Thermodynamics of the Dissolution of Borax INTRODUCTION General chemistry students are introduced to the principles of thermodynamics in lecture, but rarely have the opportunity to measure and determine the thermodynamic parameters of a chemical and/or physical process. It was with this intent that this experiment was developed and class tested. (Jo Beran Texas A&M) Data collected from the titration of a saturated borax solution prepared over a range of temperatures permits the determination of the change in enthalpy, change in entropy, and the free energy change for the dissolution of borax. The chemicals, primarily borax and hydrochloric acid, are familiar to students and safe for handling and disposal. Additionally, setting up the apparatus and the techniques for handling the saturated borax solutions over the range of temperatures provides an experiment (and an experience) that combines many of the procedures and techniques learned previously in the laboratory. Work Arrangement: Pairs of students. Several steps in the procedure require a cooperative effort. Time Requirement: On average, 2 hours to collect the data and another hour to plot the data and complete the calculations. To maximize efficiency, one pair of students can standardize the HCl solution while another pair of students can prepare the water baths and set up the borax equilibria. Together the two pairs can complete the lab and the data analysis. An option for instruction is to have the standardized HCl prepared in the stockroom—students can begin the Experimental Procedure with Part B. LECTURE OUTLINE 1. Follow the Instruction Routine outlined in “To the Laboratory Instructor.” 2. The extent to which the principles of thermodynamics are to be covered is dependent upon the students’ experiences in lecture. A discussion of the interrelationships of Equations 26.1 and 26.5 producing Equation 26.6 and its rearrangement to form Equation 26.7 is a minimal discussion. 3. Explain how the plotted data of ln Ksp vs. 1/T produces a linear relationship with a negative slope and how the values of ∆H° and ∆S° are be obtained from the data plot. 4. Review the procedure and analysis by which the Ksp of borax is determined, i.e., the concentration of the B4O5(OH)42- ion is measured by a titration with a standardized HCl solution (Equation 26.10). From the data of the titration, the Ksp is calculated. 5. Part A. The titration of a sodium carbonate primary standard is a new procedure for students. The necessity of removing the generated carbon dioxide with heat should be explained for students’ full understanding of the standardization procedure of hydrochloric acid. 6. Demonstration. Demonstrate the procedure for the titration of a warm sodium carbonate solution. 7. Several warm water baths are to be set up for the experiment. To avoid duplications and crowding, advise student pairs to share these apparatus. But, when students share apparatus, there is always the possibility of losing the identity of samples—have students mark their samples. 8. Introduce students to Excel if you haven’t already done so. The lengthy data can be analyzed and plotted using the spreadsheet—there would be fewer errors in calculations and data plots. Be careful while handling conc HCl. Dispose of the “neutralized” solutions in the “Waste Salts” container. Dispose of the standardized HCl solution in the “Waste Acids” container. Dispose of the borax solutions in the “Waste Salts” container. 1. To save time, you could prepare the standard 0.50 M HCl in the stockroom. 2. If standardizing, to expedite the procedure, the sodium carbonate can be dried in advance of the laboratory. 3. About 0.42 g of Na2CO3 is needed to react with ~16 mL of 0.50 M HCl. 4. Titrating a warm solution (and the reason for titrating the solution while warm) is a new experience for most students. Observe the students’ techniques during the titration. 5. Several warm water baths (not to exceed 60°C) at varying temperatures (need not be at a particular temperature, but rather at a carefully measured temperature) are to be set up—encourage students to share the baths, especially if hot plates are the heat source. 6. Allow the solid borax to settle, but maintaining the same temperature, before continuing to Part B.5. 7. None of the solid borax is to be transferred, only 5 mL of the saturated solution! 8. Some borax may crystallize in the pipettes from the 5-mL of solution collected at the higher temperatures when it returns to room temperature. That solid borax must be transferred to the Erlenmeyer flasks for the titration, either by heating the test tube to redissolve the borax or by using several washes in the transfer process. 9. Students following good titration technique will obtain more precise results. 10. The calculations, data plots, and interpretations of the data plots take time. I advise students to collect all of the data, review it with the me before leaving the laboratory (and initial the data to avoid data changes), and then submit the completed report at a later date. CAUTIONS & DISPOSAL TEACHING HINTS This is an opportunity to incorporate Excel (or similar spread sheet) into the data and analysis of the experiment. The graph and slope can be directly determined from the Excel data plot. Some assistance may be needed for completion of the calculations. 11. Data collected (average of repeated trials) by Mark Case Emmaus High School, during a Summer, 2005 graduate class at the Taft School in Watertown CT. CHEMICALS REQUIRED 1/T Ksp ln Ksp 276.15 3.62E-03 8.70E-04 -7.05 102.96 286.15 3.49E-03 3.10E-03 -5.78 298.15 3.35E-03 1.00E-02 -4.61 S (J/mol•K) 311.73 313.15 3.19E-03 0.11 -2.21 323.15 3.09E-03 0.66 -0.42 sodium carbonate, primary standard concentrated HCl methyl orange indicator borax bromocresol green SPECIAL EQUIPMENT H (kJ/mol) Temperature hot plates for warm water baths drying oven desiccator weighing paper or dish balance (±0.001 g) 50-mL buret, buret brush, and funnel 125 mL Elrenmeyer flasks marking pen ice thermometer, 110°C; temp probe preferred 5-mL volumetric pipettes ring stand and buret clamp G (kJ/mol) 10.06 2g dropper bottle 35 g dropper bottle 4 1 10–12 1 5 graphing software or graph paper “Waste Salts” container “Waste Acids” container . 1 2. 3. 4. Graph ln Ksp (y-axis) vs. T-1 (x-axis) A minimum of five data points is to be plotted in the experiment, but six are suggested. H° for the dissolving process is determined from the slope of the line of the graph equal to –H°/R. S° for the dissolving process is determined from the y-intercept equal to S°/R 1. Too high. The moles of Na2CO3 will be erroneously too high and therefore the calculated moles of HCl (2:1 mole ratio) will be high. As a result the reported molar concentration of HCl will be too high. 2. Too low. The borax solution is not saturated, therefore less B4O5(OH)42- will be present for the titration and less moles of B4O5(OH)42- will be analyzed. As a result, a lower molar concentration of B4O5(OH)42- will be reported and a Ksp value that will be too low. 3. The slope of a hand-drawn line may be more representative of the data because of an inherent bias for drawing a straight line that minimizes a spurious data point. A least squares program (for example, a trend line by Excel) considers all data points equally. MORE HINTS LABORATORY QUESTIONS 1. The molar solubility of a salt with the general formula, M2Q, is 2.3 x 10-3 mol/L. a. What is the Ksp of the salt? [Answer: 4.9 x 10-8] b. What is the free energy change for the dissolution of the salt at 25°C? R = 8.314 x 10-3 kJ/mol•K. [Answer: +41.7 kJ] SAMPLE LABORATORY QUIZ 2. The solubility of borax at room temperature is about 6.3 g/100 mL. Assuming the formula of borax to be Na2B4O5(OH)4•8H2O (molar mass = 381.37 g/mol), what is the molar solubility of borax and what is the Ksp of borax at room temperature? [Answer: molar solubility = 0.165 mol/L, Ksp = 1.8 x 10-2] 3. A 0.406-g sample of anhydrous sodium carbonate (molar mass = 106 g/mol) is used as a primary standard to standardize a hydrochloric acid solution. If 19.6 mL of the HCl solution is required to reach the stoichiometric point in the titration, what is the molar concentration of the HCl solution? [Answer: 0.391 M HCl] 4. From a data plot, the ∆H for the dissolution of a salt is +1.03 x 102 kJ/mol and the ∆S is + 311 J/mol•K. What is the free energy change for the dissolution of the salt at 25°C? [Answer: ∆G° = + 10.3 kJ/mol] 5. The dissolution of most salts is an endothermic processes, those that absorb energy from the surroundings. Explain why/how an endothermic process can be spontaneous. [Answer: The increase in entropy (the change in the ordering of the ions in the salt crystal to the large disorder of the ions in solution) is a driving force that overrides the nonspontaneity of the endothermic process.] 6. A 0.338 g sample of anhydrous sodium carbonate, Na2CO3, is dissolved in water and titrated to a methyl orange endpoint with 15.3 mL of a prepared hydrochloric acid solution. What is the molar concentration of the HCl solution? mol Na2CO3 2 mol HCl 0.338 g Na2CO3 x 106 g Na CO x 1 mol Na CO 2 3 2 3 [Answer: 0.0153 L = 0.417 M HCl] 7. For the analysis of the B4O5(OH)42- concentration in a saturated borax solution, suppose that the bromocresol endpoint was surpassed. How does this affect the reported solubility product of borax for that sample? Explain. [Answer: Too high. If the stoichiometric point is surpassed, a higher than actual moles of B4O5(OH)42- would be reported, resulting in a solubility product for borax to be “too high”.]