Ksp - chem212s2012
advertisement

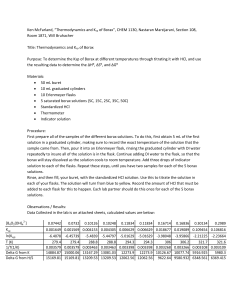
Borax Na2B4O7·10H2O (Sodium Tetraborate Decahydrate ) Determination of K, ΔG˚, ΔH˚, and ΔS˚ Quiz Question # 4 Look at following equation to write down your Ksp: 2 Na2 B4O7 10H 2O(s) 2Na (aq) B4O5 (OH )4 (aq) 8H 2O(l ) First things first… Safety: Put bags away Goggles Lab Jacket Turn in Lab Reports LAB! Borax Equilibrium 2 Na2 B4O7 10H 2O(s) 2 Na (aq) B4O5 (OH ) 4 (aq) 8H 2O(l ) Na B O (OH ) H O K 2 2 4 5 8 4 Na2 B4O7 10H 2O K Na 2 B O (OH ) H O 2 2 4 K sp Na 5 8 4 2 B O (OH ) 2 2 4 5 4 Let’s Simplify!! Stoichiometry tells us that for each Na2+ there are two B4O5(OH)42-: Na 2B O (OH ) 2 4 4 B O (OH ) K sp Na 5 2 2 4 K sp 2 B4 O5 (OH ) 4 2 5 4 B O (OH ) 2 2 4 K sp 4 B4O5 (OH ) 4 5 2 3 4 Today’s Rxn Actual reaction for today: 2 B4O5 (OH ) 4 (aq) 2HCl 3H 2O 4B(OH ) 3 (aq) 2Cl The borate ion titrated with HCl yields the above. Calculate moles borate ion to get Ksp. Gibbs Free Energy (ΔG), Enthalpy (ΔH), and Entropy (ΔS) Once you have Ksp, determine the Gibbs free energy: G RT ln K sp G H TS RT ln K sp H TS Rearrange to a linear form (so you can compare with a graph) by dividing both sides by (-RT) ln K sp H TS RT RT and T cancels out ln K sp y H 1 S R T R = m x + b Changes in Procedure Each group does one temp (assigned). 2 trials. Add 2 scoops of borax and 75 mL of distilled water to a beaker Heat the solution (<50 °C) so that the solution is saturated. Heat slowly! No need to speed up the heating process. Once slightly above assigned temperature, take off hotplate. If all the solid has dissolved, add some more borax to beaker. Pour 5 mL of the solution into a graduated cylinder once the solution is at the desired temperature. Record volume to tenths place. Be sure to record temperature to the tenths place. Procedure (continued) Add the 5 mL of solution to an Erlenmeyer with 50 mL distilled water, 5 drops bromocresol green, mix thoroughly, add a little distilled water to rinse the graduated cylinder into the Erlenmeyer (Quantitative Transfer) Titrate with HCl (clean/rinse buret and fill w/ ~20 mL HCl) REPEAT! The above steps for two more titrations. DO NOT try to prepare three samples at once, unless you can guarantee the temperature of the borax solution will not change dramatically! Turn in class: Pre-lab, filled in data tables, observations Type in you and your partner’s data in class computers on opened excel spreadsheet Name [HCl] Jack and Jill (M) 0.250 Trial 1 Volume Borax Volume Temp Solution Used HCl used (°C) (mL) (mL) 33 5.2 5.9 Formal Lab Report – due in two weeks Table 1, 2 – for each trial solve for: Temp (K) mol HCl mol borate [Borax] used ion (M) Ksp ΔG lnKsp (kJ/mol ) Table 4 – average the trials to find (for each temp): Temp (K) Ksp ΔG lnKsp (kJ/mol ) 1/T Data Tables (con’t) Table 5 – use ΔH and ΔS to solve for ΔG for each Temp ΔG Temp (K) (kJ/mol) Graph: Make using Table 4 data Graph 1: 1/T vs. ln Ksp 0.00 0.00315 -1.00 0.0032 0.00325 0.0033 0.00335 0.0034 ln Ksp -2.00 y = 461.3x - 7.0772 R² = 0.0006 -3.00 -4.00 Series2 -5.00 Linear (Series2) -6.00 -7.00 -8.00 -9.00 1/T (1/K) Calculations Find molarity of borax (mol/L) [Borax] Find moles borate (use moles HCl) Find volume of solution (borate + HCl) Find Ksp Ksp = 4[Borax] Calculations Determine dH (kJ/mol) and dS (J/mol K) from this graph. note: dH = ΔH Use trendline from graph y = mx + b H ln K sp R 1 S T R Calculations con’t Calculate the values for dG for each sample using: dG = -RT ln Ksp used for table 4 dG = dH - TdS with your solved values for dH and dS used for table 5 Discussion Analysis Consider the following: Compare solving delta G between the two equations, which yielded better results? Consider the precision of the graphs