Lab 2: Super-Critical Fluid Extraction (SFE)
advertisement

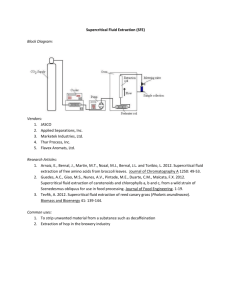
Instrumental Analytical Chemistry Lab 2: Supercritical Fluid Extraction INTRODUCTION The supercritical fluid extractor is an instrument that is ideal for extracting organics or various compounds. The most common super critical fluid is CO2. A substance is considered a “supercritical fluid” when it’s above the ideal critical temperature and pressure. Super critical fluids have diffusivities a 10 fold higher than that of liquids and can handle unstable compounds better. Also, the mass transfer between the supercritical fluid and an organic compound within a solid matrix is very simple and is not hard to do. Thus, many organics compounds can be extracted with a few hours to minutes. Using the supercritical fluid extraction CO 2 to extract organic compounds leads to no hazardous bi-products, which makes this method relatively green. SFE has its setbacks. CO2 has a very low polarity which means that it cannot extract polar substance very efficiently. However, it can extract different non-polar compounds very efficiently. If a polar compound is desired to be extracted, a polar additive has o be added to the system to aide in extraction. In this lab we will be exploring the SFE and running various known and unknown samples of wax. Extraction efficiencies will be calculated to see how well the extraction process was done. PURPOSE: the purpose of this lab is to understand and use the SFE to extract various samples of known and unknown amounts of wax. Also, the extraction efficiencies will be calculated to see how efficient the extracting process was. Based on the knowledge learned in this experiment and some additional research two additional questions will be answered to pull the aspects of this lab together. PROCEDURE 1. 2. 3. 4. 5. 6. Set-up SFE to the following condition: 100⁰C oven temperature, 100⁰C valve, and 300bar. Massed out 3 samples of Solo wax and recorded the three masses. Massed three Erlenmeyer flasks with paraffin paper and recorded the weights. Pack the column with glass wool layer followed by sample layer and another layer of glass wool. Ran three samples of wax for 30 minutes and calculate the extract efficiencies. Samples were dispensed from instrument into the appropriate flask. **Repeated steps 2-6 for a known amount of wax paper and the unknown sample. DATA Trial (Time Ran) 1 (30min) 2 (30min) 3 (00min) MASSES OF SOLO WAX SAMPLES Mass of wax (g) Mass of flask (g) 1.0140g 79.0794g 1.0085g 87.9418g 1.00333g 95.5067g Final Mass of Flask (g) 79.1315g 88.9498g (Sample wasn’t done) CALCULATIONS Extraction Efficiency: Trial 1 78.0794𝑔 𝑥 100 = 98.6% 79.1315𝑔 Extraction Efficiency: Trial 2 87.9418g 𝑥 100 = 98.8% 88.9418g Only two samples were run. We didn’t’ have enough time to run the third sample massed out because we ran out of time. Trial (Time Ran) 1 (30min) Unknown B 2 (30min) Known # 05 MASSES OF Known & Unknown WAX SAMPLES Mass of wax (g) Mass of flask (g) 0.5765g 77.2019g 0.5761g 82.6071g Final Mass of Flask (g) 77.3470g 82.7120g CALCULATION Extraction Efficiency unknown B 77.3470𝑔 − 77.2019𝑔 = 0.1451𝑔 0.1451𝑔 𝑥 100 = 25.2% 0.5765𝑔 Extraction Efficiency Known #5 82.7120𝑔 − 82.6071𝑔 = 0.1049𝑔 0.1049𝑔 𝑥 100 = 18.20% 0.5761𝑔 RESULTS We started out running two samples of solo wax to see how well the extraction efficiency was for the instrument. We ran two samples and the extraction efficiency were in the 98% tile. They were 98.6% and 98.8% respectively. Thus, upon good results we ran a known and a unknown sample. The known sample had a wax content of about 15.2%. Our result for the known sample was an extraction efficiency of 18.20%. The unknown that was ran had an extraction efficiency of 25.2% CONCLUSION Our sample runs analysis can out well. We had a 98.8% extraction. This let us know that we did a good job preparing and analyzing the samples of solo wax. Thus, our instrument understudy, was well keep and cleaning property to have provide good yields. We ran a sample that was known and an unknown sample. The sample that was known was suppose to have a wax content of 15.2% but our records indicated that that paper contained 18.25 wax. We were off by about 3 units. This could be from not letting the previous samples run long enough and that could have added to the mass. I think the only problem that could have contributed to the error in our reading was the weighting and some paraffin, which we used to cover the output site and flask, could have melted. Mainly, it could have been a measuring error, because we weren’t the only people that weighting the known sample and when that sample was initially prepared and tested, the instrument could have had different setting or the weighting could have been done differently, thus their extraction efficiency may not have been as good or different from ours. However, I think our analysis was pretty accurate, but better technique could have improved our results. Therefore, I do feel confident in saying that our unknown sample was 25% wax because given the extraction efficiency the majority of the procedure, and the data looked well. Plus, we experienced little to no problem because our labs with this instrument went very smoothly once the instrument was setup and running. QUESTIONS (1) The extraction efficiency increases when the density of CO2 increase because when you increase the density it causes the super-critical fluid to act more like a liquid and behaving more like a liquid will help extraction more product because more mobile phrase-stationary phrase interactions occur. (2) As you increase the temperature of the system the extraction efficiency will increase as well because the density will increase, which contribute to better extraction.