standard electrode potentials (e æ )
advertisement

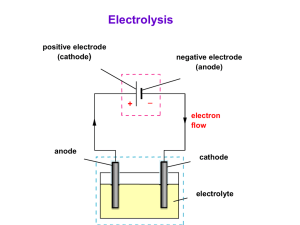
ELECTROCHEMISTRY Electrical Energy ↔ Chemical Energy REDUCTION, OXIDATION and REDOX REACTIONS A redox reaction is one where there is a transfer of one or more electrons from one atom or ion to another. Oxidation: this is the loss of one or more electrons from an atom or ion OIL Reduction: this is the gaining of one or more electrons by an atom or ion RIG In a REDOX reaction one species is reduced (gains one or more electrons, or oxidation number decreases) and another is oxidised (loses one or more electrons, or oxidation number increases). Eg: 2Mg + O2 2MgO magnesium is oxidised - the oxidation half-reaction is: Mg Mg2+ + 2e- (ox no.: 0 to +2; ie an increase) oxygen is reduced - reduction half-reaction is: O2 + 2e- O2- (ox no.: 0 to -2; ie a decrease) Oxidising agent: an oxidising agent oxidises another substance by accepting one or more electrons and being reduced itself. e.g. oxygen in above reaction Reducing agent: a reducing agent reduces another substance by donating one or more electrons and being oxidised itself. e.g. magnesium in above reaction In the data booklet you have two tables. . They give a number of halfreactions and are written as reductions. The reverse of each is an oxidation half reaction. F2 is the most powerful oxidising agent and Li is the most powerful reducing agent on the table. Galvanic Cell Electrolytic Cell Energy chemical to electrical electrical to chemical Cathode Positive (reduction) Negative (reduction) Anode Negative (oxidation) Positive (oxidation) GALVANIC (or VOLTAIC) CELLS An electrochemical cell that derives electrical energy from a spontaneous redox reaction taking place within the cell. In electrochemical cells we take a redox reaction and arrange the equipment so that the oxidation and reduction take place in different locations and the electron transfer takes place through an external wire. If the two half reactions are in different containers then we join the two containers by a SALT BRIDGE. A salt bridge is made up of a concentrated solution of salt (usually KCl or KNO3) and it keeps the solutions neutral by the diffusion of oppositely charged ions. At one electrode oxidation occurs (the ANODE) which produces electrons. The electrode will be negative. e.g. Zn → Zn2+ + 2eAt the other electrode reduction occurs (the CATHODE) which uses up electrons. The electrode will be positive. e.g. Cu2+ + 2e- → Cu The result is a potential difference (emf) between the electrodes and work can be done. A current will flow if the two electrodes are connected by a conductor. The Cell Reaction will be Zn + Cu2+ Zn2+ + Cu FACTORS WHICH AFFECT THE EMF OF A CELL 1. Which half-cells are used. e.g. Mg/Mg2+//Cu2+/Cu has a higher emf than Zn/Zn2+//Cu2+/Cu 2.Concentrations of solutions / pressure of gases. 3.Temperature (usually higher T lower emf) Cell reactions are actually reversible reactions and with a high resistance voltmeter attached equilibrium is nearly reached. If the change in temperature or concentration causes a shift to the right in equilibrium then the emf will increase. e.g Mg + Cu2+ Mg2+ + Cu ΔH<0 An increase in temperature will _______________ the emf. Decreasing [Cu2+] will _______________ the emf. An increase in pressure will _______________ the emf. e.g. H2(g) + Cu2+(aq) 2H+(aq) + Cu(s) + heat A decrease in temperature will _______________ the emf. A decrease in pressure of H2 will _______________ the emf. The addition of an alkali will _______________ the emf. TYPICAL CELL DIAGRAM: V Mg KNO3 MgSO4 Oxidation: Cu CuSO4 Mg → Mg2+ + 2e- Reduction: Cu2+ + 2e- → Cu The cell reaction: Mg + Cu2+ → Mg2+ + Cu Electrons go from Mg to Cu through wire. Mg2+ ions flow through salt bridge to right. SO42- ions to left. CELL NOTATION – conventional notation for representing electrochemical cells 1. Anode on left. 2. Concentrations of ions should be put in (1.0 mol.dm-3 under standard conditions). 3. Salt bridge shown as || 4. Platinum is used if there is no metal in a half-cell reaction. e.g. For above cell at standard conditions Mg | Mg2+(1,0 mol.dm-3) || Cu2+(1,0 mol.dm-3) | Cu Electron flow is thus always from LEFT to RIGHT STANDARD ELECTRODE POTENTIALS (E) Also called REDUCTION POTENTIALS - given for each reduction halfreaction. Reduction potentials were determined by attaching each half-cell to a STANDARD HYDROGEN ELECTRODE as a reference electrode, using a salt bridge, and recording the voltage across them. A standard hydrogen electrode consists of a platinum electrode in a solution of acid with a [H+] of 1 mol.dm-3 over which hydrogen at 1 atm is bubbled. All are at 25oC. H2 H2 Pt electrode Hydrogen gas (1 atm) 2H+ + 2e- ⇌ H2 E = 0,00 V Acid with [H+] = 1,0 mol.dm-3 The standard hydrogen electrode is always attached to the negative terminal of the voltmeter then the reading and sign on the voltmeter will be the standard electrode potential for the other half-cell. Meaning of Standard electrode potentials: In a redox system, there is competition for electrons. The E value indicates how successful substances are in gaining electrons, or reduction. - more positive E value greater tendency to undergo reduction (proceed in forward direction as written in tables) - less positive E value greater tendency to undergo oxidation (proceed in reverse direction as written in tables) USES OF STANDARD REDUCTION POTENTIALS 1. Calculating emf under standard conditions Ecell = Ecathode - Eanode Ecell = Eoxidising agent - Ereducing agent e.g. What is the emf of the following cell under std conditions? Fe/Fe2+//Cl/Cl2,Pt Ecell = Ecathode - Eanode = 1,36 – (-0,44) = 1,80 V e.g. What is the emf for the cell with cell reaction: 2Ag+ + Pb → 2Ag + Pb2+ (std conditions) Ecell = Eoxidising agent - Ereducing agent = = 2. Seeing if a reaction will occur spontaneously. A reaction will occur spontaneously if its Ecell is positive. ( Note: this is only strictly true for standard conditions. e.g. Will Ni2+ ions be reduced spontaneously by Cu metal? Ni2+ + Cu → Ni + Cu2+ Ecell = Ecathode - Eanode = = Generally, if the half-reaction with the more positive E value is reduced, the reaction will be spontaneous. e.g. An unknown half-cell is connected to a Zn/Zn2+ half-cell (all under standard conditions) and the emf was measured as 0,63 V. If the Zn/Zn2+ was the anode, then: a) What was the metal in the other half-cell? b) What is the cell reaction? GALVANIC CELLS GOING FLAT. A cell is flat when, when connected up so a current could flow, chemical equilibrium exists at each electrode. Consider the Cu/Zn example: If the cell is not connected up by the wire then the following chemical equilibrium exists at each electrode: Zn: Zn ⇌ Zn2+ + 2e- Cu: Cu ⇌ Cu2+ + 2e- When the electrodes are connected by the wire this equilibrium is immediately broken in the following way: Zn: The Zn Zn2+ + 2e- reaction rate increases Cu: The Cu2+ + 2e- Cu reaction rate increases. Thus the overall cell reaction is: Zn + Cu2+ Zn2+ + Cu and a current flows. As the current flows, therefore, the concentration of Zn2+ ions increases and the Cu2+ ions concentration drops. This causes the Cu2+ + 2e- Cu reaction rate to drop in one half-cell and the Zn2+ + 2e- Zn reaction rate to rise in the other. As a result a new chemical equilibrium eventually results in each half-cell (Zn ⇌ Zn2+ + 2e- and Cu ⇌ Cu2+ + 2e-), the current stops flowing and the cell is said to be FLAT. ELECTROLYTIC CELLS Use electrical energy to bring about chemical change (decomposition). In electrolytic cells: - electrodes are connected to a direct current and dipped into the liquid electrolyte - electrons flow from the negative terminal of the battery, around the external circuit and back to the positive terminal. - positive and negative ions carry the current in the electrolyte Electrolysis of molten ionic compounds Na+ ions migrate toward the cathode, where they are reduced to sodium metal. Anode: 2 Cl-(l) Cl2(g) + 2e- Similarly, Cl- ions migrate to the anode and are oxided to form chlorine gas. Cathode: Na+(l) + e- Na(l) Overall: 2Na+(l) + 2Cl-(l) 2Na(l) + Cl2(g) Cl2 gas can be collected surrounding the cell. Na metal is less dense than the molten salt and is removed as it floats to the top of the container. Electrolysis of aqueous solutions of ionic compounds The electrolysis involves decomposition, but the products may differ because water itself produces ions (H+ and OH-) and these can undergo oxidation and reduction. Electrolysis of concentrated CuCl2 Anode: chlorine gas (yellow-green) 2Cl-(aq) Cl2(g) + 2eCathode: copper (Brown) copper(II) chloride Cu2+(aq) + 2e- Cu(s) solution (blue or green) Overall: e- NB NOTE: Some aqueous solutions are more complicated Ions of Active Metals resist reduction at cathode in aqueous solutions Examples: Na+, K+, Li+, Ca2+, Ba2+ Instead: 2H2O(l) + 2e- H2(g) + 2OH-(aq) happens each time Polyatomic anions resist oxidation at anode in aqueous solutions Examples: NO3-, SO42-, CO32-, PO43- etc. Instead: 2H2O O2 + 4H+ + 4e- happens each time EXAMPLES: Electrolysis of KNO3(aq) Anode: Cathode: Overall: Electrolysis of HCl(aq) Anode: Cathode: Overall: Electrolysis of CuBr2(aq) Anode: Cathode: Overall: Electrolysis of KI(aq) Anode: Cathode: Overall: APPLICATIONS OF ELECTROLYSIS REACTIONS ELECTROPLATING Unreactive metals (Cu, Ag, Ni, Au) can be plated onto objects by the electrolysis of a concentrated aqueous solution of the metal’s salt (ELECTROLYTE). The object to be plated is made the NEGATIVE electrode (cathode). The metal being plated is made the POSITIVE electrode (anode). It dissolves during the process thus keeping the metal ion concentration constant. (It requires less energy to oxidise the metal atoms into cations than oxidise the anions present.) Example: PLATING WITH SILVER. Electrolyte – silver nitrate solution Anode – silver metal Cathode – object to be plated Oxidation half-reaction: Ag(s) Ag+(aq) + eReduction half-reaction: Ag+(aq) + e- Ag(s) REFINING OF COPPER In the final part in the production of pure copper, electrolysis is used turn impure copper into pure copper. Electrolyte: copper sulphate solution (acidified) Anode: impure copper Cathode: pure copper Oxidation half-reaction: Cu(s) Cu2+(aq) + 2eReduction half-reaction: Cu2+(aq) + 2e- Cu(s) The impure anode dissolves and the copper ions formed transverse the cell to the cathode and plate it. Impurities fall to the bottom of the cell. THE CHLOROALKALI INDUSTRY. The electrolysis of saturated sodium chloride (brine) produces chlorine, sodium hydroxide and hydrogen. The electrolytic process is expensive (due to the amount of electricity used) but the process is economic due to the large number of uses of the products. Definition of electrolysis: The chemical process in which electrical energy is converted to chemical energy OR the use of electrical energy to produce a chemical change. Electrolysis takes place in an electrolytic cell. The overall reaction is 2NaCl (aq) + H2O (l) → 2NaOH (aq) + Cl2 (g) + H2 (g) The electrolysis of sodium chloride can take place in three types of cells, namely the membrane cell, the diaphragm cell or the mercury cell. The latter two are being phased out due to environmental risks associated with them. Consider the membrane cell. The anode is made from titanium and the cathode of nickel or steel. An ion-exchange membrane up the middle lets sodium ions through to the cathode but keeps the gases apart. As a result, the NaOH only forms in the cathode compartment and does not mix with the NaCl. The membrane is a polymer impregnated with negative charges that help draw positive charges (Na+) through it but repel negative charges. Na+ Hydrogen is produced at the cathode from water: Half reaction:- 2H2O + 2e- H2 + 2OHChlorine is produced at the anode: Half reaction - 2Cl- Cl2 + 2e- There are no environmental risks associated with the membrane cell. Care just needs to be taken when handling the products. The mercury cell poses problems due to the highly poisonous nature of mercury and it is inefficient. The diaphragm cell uses asbestos as its diaphragm, and this also has documented risks related to breathing and cancer. However, in all cells the electricity required needs to be generated and this may well result in pollution and the production of greenhouse gases – especially if coal is used. Uses of chlorine, sodium hydroxide and hydrogen: Chlorine: Properties: Yellow-green gas. 2x denser than air, Poisonous. Uses: In the preparation of PVC, solvents (eg trichloroethane for dry cleaning), paints and dyes, hydrochloric acid, bleaches, disinfectants, and pesticides, Also use in water purification – killing bacteria in tap water and swimming pools. Sodium hydroxide: Properties: Strong alkali. “Caustic soda” – corrosive. Very soluble in water. Uses: In the preparation of soaps, detergents, paper, textiles. Drain cleaner. Hydrogen: Properties: Colourless gas. Lightest gas. Insoluble in water. Uses: In the preparation of nylon, hydrogen peroxide, margarine. ALUMINIUM PRODUCTION This page starts by looking at the extraction of aluminium from its ore, bauxite, including some economic and environmental issues. It finishes by looking at some uses of aluminium. Extracting aluminium from bauxite Aluminium is too high in the electrochemical series (reactivity series) to extract it from its ore using carbon reduction. Instead, it is extracted by electrolysis. The ore is first converted into pure aluminium oxide by the Bayer Process, and this is then electrolysed in solution in molten cryolite - another aluminium compound. (The aluminium oxide has too high a melting point to electrolyse on its own.) The usual aluminium ore is bauxite. Bauxite is essentially an impure aluminium oxide. Bauxite is purified by the Bayer Process which uses NaOH. The result is pure Al2O3. Conversion of the aluminium oxide into aluminium by electrolysis The aluminium oxide is electrolysed in solution in molten cryolite, Na3AlF6. The diagram shows a simplified version of an electrolysis cell. The cell operates at a low voltage of about 5 - 6 volts, but at huge currents of 100,000 amps or more. The heating effect of these large currents keeps the cell at a temperature of about 1000°C. Aluminium is released at the cathode. Aluminium ions are reduced. Al3+ + 3e- Al Oxygen is produced initially at the anode. 2O2- O2 + 4eHowever, at the temperature of the cell, the carbon anodes burn in this oxygen to give carbon dioxide and carbon monoxide. Continual replacement of the anodes is a major expense. Economic considerations (read through – no need to learn in detail) 1. 2. 3. The high cost of the process because of the huge amounts of electricity it uses. Energy and material costs in constantly replacing the anodes. The huge use of electricity in South Africa in Aluminium production causes shortages in electricity for the rest of the country. In addition the electricity is sold to the industry at below cost and this is recouped from other consumers. Environmental problems in mining and transporting the bauxite 1. 2. Loss of landscape due to mining, processing and transporting the bauxite. Noise and air pollution (greenhouse effect, acid rain) involved in these operations. Extracting aluminium from the bauxite 1. 2. 3. 4. 5. Loss of landscape due to the size of the chemical plant needed, and in the production and transport of the electricity. Noise. Atmospheric pollution from the various stages of extraction. For example: carbon dioxide from the burning of the anodes (greenhouse effect) Pollution and greenhouse gases caused by power generation (varying depending on how the electricity is generated.) Disposal of red mud into unsightly lagoons. Recycling Recycling aluminium uses only about 5% of the energy used to extract it from bauxite. Aluminium is used for: Because: aircraft light, strong, resists corrosion other transport such as ships' superstructures, container vehicle bodies, tube trains (metro trains) light, strong, resists corrosion overhead power cables (with a steel core to strengthen them) light, resists corrosion, good conductor of electricity saucepans light, resists corrosion, good appearance, good conductor of heat .