Physics 410 Spring 2008 HW#1
advertisement
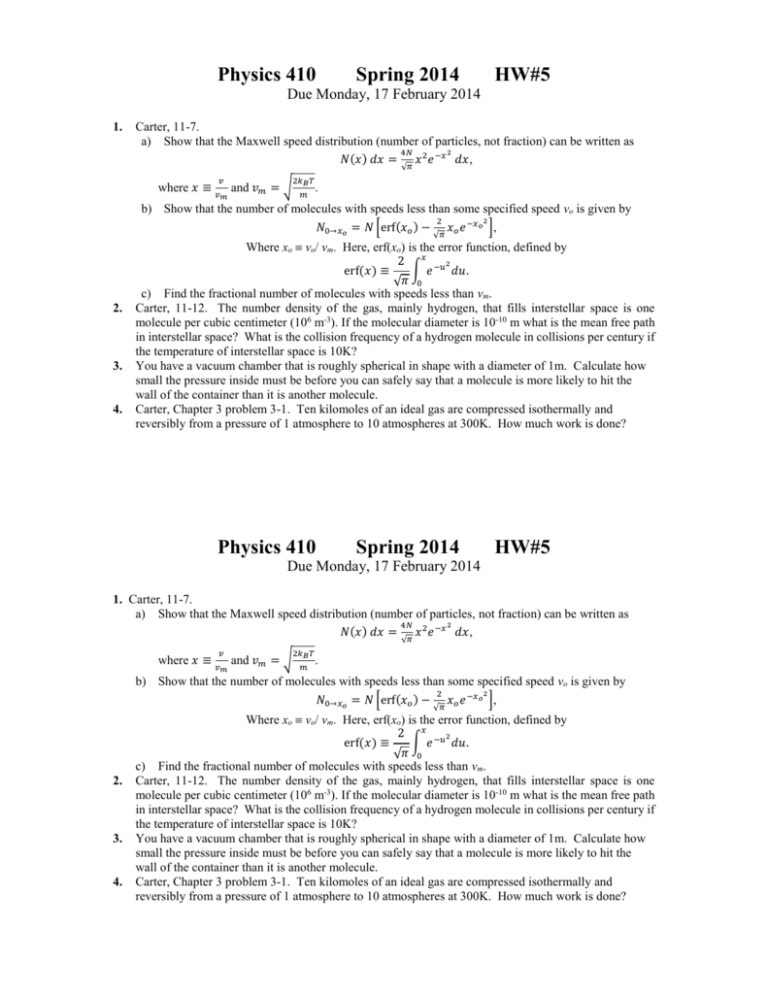
Physics 410 Spring 2014 HW#5 Due Monday, 17 February 2014 1. Carter, 11-7. a) Show that the Maxwell speed distribution (number of particles, not fraction) can be written as 2 4𝑁 𝑁(𝑥) 𝑑𝑥 = 𝑥 2 𝑒 −𝑥 𝑑𝑥, √𝜋 where 𝑥 ≡ 𝑣 𝑣𝑚 2𝑘𝐵 𝑇 and 𝑣𝑚 = √ 𝑚 . b) Show that the number of molecules with speeds less than some specified speed vo is given by 2 2 𝑁0→𝑥𝑜 = 𝑁 [erf(𝑥𝑜 ) − 𝑥𝑜 𝑒 −𝑥𝑜 ], √𝜋 2. 3. 4. Where xo vo/ vm. Here, erf(xo) is the error function, defined by 2 𝑥 −𝑢2 erf(𝑥) ≡ ∫ 𝑒 𝑑𝑢. √𝜋 0 c) Find the fractional number of molecules with speeds less than vm. Carter, 11-12. The number density of the gas, mainly hydrogen, that fills interstellar space is one molecule per cubic centimeter (106 m-3). If the molecular diameter is 10-10 m what is the mean free path in interstellar space? What is the collision frequency of a hydrogen molecule in collisions per century if the temperature of interstellar space is 10K? You have a vacuum chamber that is roughly spherical in shape with a diameter of 1m. Calculate how small the pressure inside must be before you can safely say that a molecule is more likely to hit the wall of the container than it is another molecule. Carter, Chapter 3 problem 3-1. Ten kilomoles of an ideal gas are compressed isothermally and reversibly from a pressure of 1 atmosphere to 10 atmospheres at 300K. How much work is done? Physics 410 Spring 2014 HW#5 Due Monday, 17 February 2014 1. Carter, 11-7. a) Show that the Maxwell speed distribution (number of particles, not fraction) can be written as 2 4𝑁 𝑁(𝑥) 𝑑𝑥 = 𝑥 2 𝑒 −𝑥 𝑑𝑥, √𝜋 where 𝑥 ≡ 𝑣 𝑣𝑚 2𝑘𝐵 𝑇 and 𝑣𝑚 = √ 𝑚 . b) Show that the number of molecules with speeds less than some specified speed vo is given by 2 2 𝑁0→𝑥𝑜 = 𝑁 [erf(𝑥𝑜 ) − 𝑥𝑜 𝑒 −𝑥𝑜 ], √𝜋 2. 3. 4. Where xo vo/ vm. Here, erf(xo) is the error function, defined by 2 𝑥 −𝑢2 erf(𝑥) ≡ ∫ 𝑒 𝑑𝑢. √𝜋 0 c) Find the fractional number of molecules with speeds less than vm. Carter, 11-12. The number density of the gas, mainly hydrogen, that fills interstellar space is one molecule per cubic centimeter (106 m-3). If the molecular diameter is 10-10 m what is the mean free path in interstellar space? What is the collision frequency of a hydrogen molecule in collisions per century if the temperature of interstellar space is 10K? You have a vacuum chamber that is roughly spherical in shape with a diameter of 1m. Calculate how small the pressure inside must be before you can safely say that a molecule is more likely to hit the wall of the container than it is another molecule. Carter, Chapter 3 problem 3-1. Ten kilomoles of an ideal gas are compressed isothermally and reversibly from a pressure of 1 atmosphere to 10 atmospheres at 300K. How much work is done?