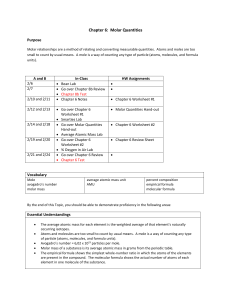
Hareem Shahzad Day 49: The Mole Quick Overview of the Lesson

Hareem Shahzad
Day 49: The Mole
Quick Overview of the Lesson:
The Mole – Pea Story
PowerPoint on the Mole and Molar Mass
Handouts : The Mole , Avogadro’s Number, Moles and Molar Mass
Textbook: Read pages 266 – 274 (Avogadro’s Number and Molar Mass)
Homework: Finish all the worksheets and answer questions from the textbook (Pg. 256 #1-15,
Pg. 275 #1)
What is a mole?
A mole is a counting unit, similar to grams, kilometres, litres, etc. Its value is 602 billion trillion or 6.02 x
10 23 in scientific notation. This number was named after Amedeo Avogadro (1776-1856). He studied gases and their quantities and he discovered that no matter what the gas was, there were the same number of molecules present in it. A mole can also be referred to as Avogadro’s Number.
A mole can be used to state the quantity of anything. The quantities we will be concentrating on will mainly include particles such as atoms, ions, and molecules.
An example of how moles are used is:
A mole of particles = 6.02 x 10 23 particles
Atomic Mass:
Atomic mass can be associated with mass in grams. For example, 1 g of H-1, 12g of C-12, and 23 g of Na-
23 have 6.02 x 10 23 atoms.
The symbol for a mole is ‘mol’ and it is used in equations whereas ‘mole’ is used in writing.
The mole is a very large unit, so it’s used to count very small things, like atoms. We also cannot actually count a mole of something so to find out whether or not we have a mole of something we determine the mass and relate that to the number of atoms present.
It is possible to know the weight of an atom in grams. However, there are problems associated with it.
1.
It isn’t easy to converts atomic mass into grams.
2.
Atoms cannot be weighed since they’re too small.
Molar Mass:
Molar mass is the mass of 1 mole of a pure substance, whether it’s an element or a compound.
The atomic mass is the mass of 1 atom of an element in atomic mass units.
The molar mass is also equal to 1 mole of atoms measured in grams.
Hareem Shahzad
Atomic Mass vs. Molar Mass:
Mass of 1 atom of Pb = 207.2 amu Mass of 1 mole of Pb atoms = 207.2 g
Mass of 1 atom of N = 14.01 amu Mass of 1 mole of N atoms = 14.01 g
*THE NUMERICAL VALUE OF THE ATOMS AND MOLES OF ATOMS IS THE SAME, ONLY THE UNITS ARE
DIFFERENT. THIS APPLIES TO MOLECULAR AND IONIC COMPUNDS AS WELL*
Molar Mass for Compounds:
How to find the Molar Mass:
1.
Write the CORRECT formula for the compound.
2.
Look up the atomic mass of each element in the compound.
3.
Multiply the atomic mass by the subscripts, if any.
4.
Add all the masses of elements together and use the unit g/mol (grams per mole)
Conversions:
molar mass Avogadro’s Number
Grams < ------------------------------------------- > Moles < -----------------------------------------> Particles
We cannot directly convert grams into particles and vice versa. It is necessary to convert the particles into moles and then grams. There is a triangle that can be used to assist us in determining how to go from one to the other.
Another way to state this is: mol = mass so n = m
g grams/mole MM mol g/mol
1.
If we are given the number of grams of a compound, we can determine the number of moles, and vice versa.
2.
In order to convert from one to the other you must first calculate the molar mass.
Video to help you better understand the mole: http://www.youtube.com/watch?v=AsqEkF7hcII
Websites to help you better understand the mole: http://crescentok.com/staff/jaskew/isr/chemistry/class13.htm
https://www.khanacademy.org/science/mcat/physical-processes/stoichiometry/v/the-mole-andavogadro-s-number http://www.chem.wisc.edu/deptfiles/genchem/sstutorial/Text4/Tx44/tx44.html
http://en.wikibooks.org/wiki/Introductory_Chemistry_Online/Mole_and_Measurement