Conductometric Titration Lab: Strong & Weak Acids
advertisement
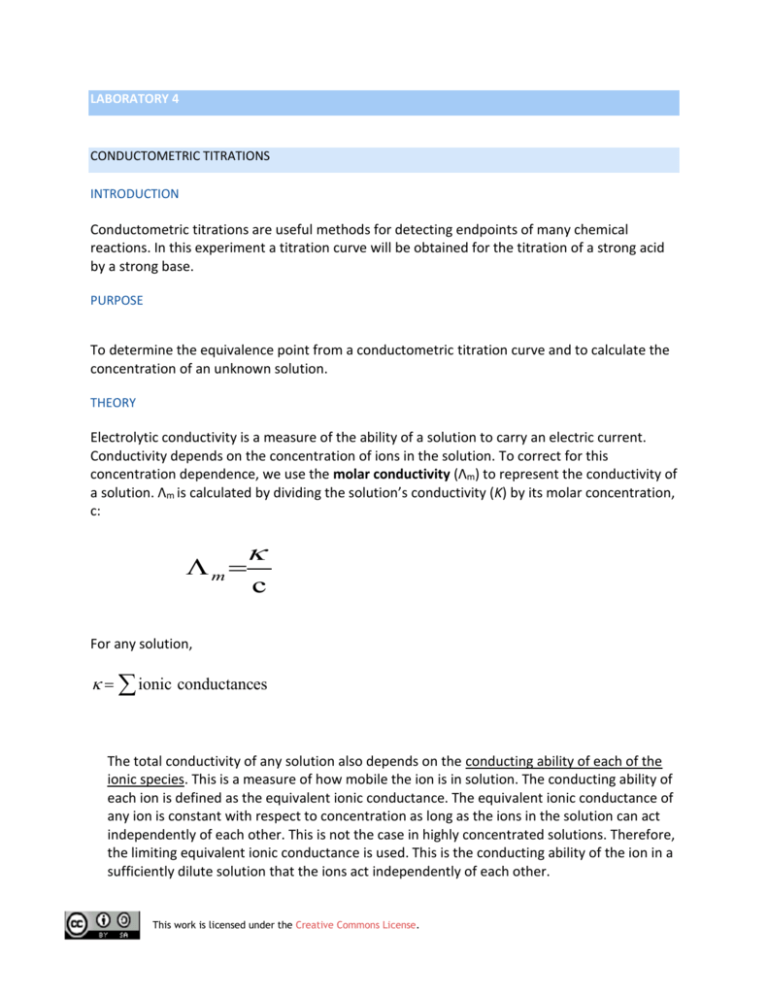
LABORATORY 4 CONDUCTOMETRIC TITRATIONS INTRODUCTION 2 Conductometric titrations are useful methods for detecting endpoints of many chemical reactions. In this experiment a titration curve will be obtained for the titration of a strong acid by a strong base. PURPOSE To determine the equivalence point from a conductometric titration curve and to calculate the concentration of an unknown solution. THEORY Electrolytic conductivity is a measure of the ability of a solution to carry an electric current. Conductivity depends on the concentration of ions in the solution. To correct for this concentration dependence, we use the molar conductivity (Λm) to represent the conductivity of a solution. Λm is calculated by dividing the solution’s conductivity (K) by its molar concentration, c: m c For any solution, ionic conductances The total conductivity of any solution also depends on the conducting ability of each of the ionic species. This is a measure of how mobile the ion is in solution. The conducting ability of each ion is defined as the equivalent ionic conductance. The equivalent ionic conductance of any ion is constant with respect to concentration as long as the ions in the solution can act independently of each other. This is not the case in highly concentrated solutions. Therefore, the limiting equivalent ionic conductance is used. This is the conducting ability of the ion in a sufficiently dilute solution that the ions act independently of each other. This work is licensed under the Creative Commons License. The Limiting equivalence ionic conductance defines the conducting ability of an ion. Of all cations, H+ has the highest mobility. Of all anions, OH- has the highest mobility. PROCEDURE 1.1: Titration of a strong acid by a strong base • • • • • Dispense 10.00 mL of standardized hydrochloric acid into 100 mL beaker and dilute to a total volume of approximately 100 mL with distilled water. Stir the solution in the beaker. Dip the conductivity cell into the solution; measure and record the conductivity of the solution. Add 1.00 mL of NaOH (~ 0.1 M) to the beaker. Stir the solution. Read and record the conductivity of solution after about 35 seconds. Continue the process until 20.00 mL of NaOH has been added. DATA AND RESULTS: • • • • Plot conductance (Y-axis) vs. mL of NaOH for the data obtained. The equivalence point for the strong acid strong base titration is found when the minimum conductivity is measured; explain why? What conductive species are present at the equivalence point? Using the data obtained from your graph, calculate the molarity of the NaOH solution. STRONG ACID (HCl) AND STRONG BASE (NaOH) The observed shape of the titration curve (1) can be explained as follows: HCl is a strong acid and thus it will be fully ionized into H+ and Cl- in solution. The high initial conductance is due to mainly H+. As NaOH (a strong electrolyte) is added, the following reaction occurs H+ (aq) + Cl- (aq) + Na+ (aq) + OH- (aq) H2O (l) + Na+ (aq) + Cl- (aq) Overall, this has the effect of replacing the high conducting ability of H+ with the less conducting Na+. Thus the overall conductance of the solution drops. This work is licensed under the Creative Commons License. At the equivalence point, the conductance is a minimum. After this point, all you are doing is adding additional OH- and Na+ to the solution and thus the conductivity rises again though at a slower rate. WEAK ACID (ACETIC ACID) AND STRONG BASE (NaOH) Acetic acid is a weak acid (& weak electrolyte) as shown by the following equation. CH3CO2H (aq) H+ (aq) + CH3CO2- (aq) where Ka is the acid-dissociation constant. Thus the majority of the acetic acid is present as non-ionised CH3CO2H Initially the conductance of the acetic acid will be lower than that of the same concentration of a strong acid as we have less H+ in solution. PROCEDURE 1.2.: Titration of a weak acid by a strong base • • • • Repeat procedure 1.1 with 10.00 mL of 0.100 M acetic acid and 0.100 NaOH. Obtain the titration curve using data collected. Compare the titration curve obtained in 1.1 and 1.2. Why is the initial conductivity of the acidic acid much lower than that of hydrochloric acid solution even though they have the same molarity? This work is licensed under the Creative Commons License.











